
[Ń”×öĢā”Ŗ”ŖÓŠ»ś»Æѧ»ł“”]
øß·Ö×Ó²ÄĮĻPET¾Ūõ„Ź÷Ö¬ŗĶPMMAµÄŗĻ³ÉĀ·ĻßČēĻĀ£ŗ
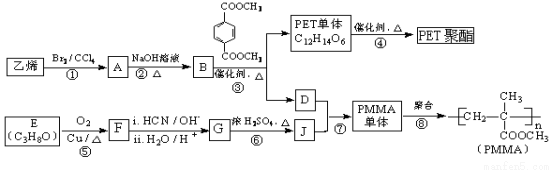
ŅŃÖŖ£ŗ
¢ń. RCOOR”ä+ R”å18OH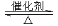 RCO18OR”å+R”äOH£ØR”¢R”䔢R”å“ś±ķĢž»ł£©
RCO18OR”å+R”äOH£ØR”¢R”䔢R”å“ś±ķĢž»ł£©
¢ņ. 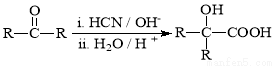 £ØR”¢R”ä“ś±ķĢž»ł£©
£ØR”¢R”ä“ś±ķĢž»ł£©
£Ø1£©GµÄ¹ŁÄÜĶÅĆū³ĘŹĒ___________”¢____________”£
£Ø2£©¢ŚµÄ»Æѧ·½³ĢŹ½ĪŖ ”£
£Ø3£©¢ŽµÄ·“Ó¦ĄąŠĶŹĒ___________________________”£
£Ø4£©JµÄijÖÖĶ¬·ÖŅģ¹¹ĢåÓėJ¾ßÓŠĻąĶ¬¹ŁÄÜĶÅ£¬Ęä½į¹¹¼ņŹ½ŹĒ ”£
£Ø5£©PMMAµ„ĢåµÄ½į¹¹¼ņŹ½ĪŖ____________________”£
£Ø6£©ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ________£ØĢī×ÖÄøŠņŗÅ£©”£
a£®¢ßĪŖõ„»Æ·“Ó¦
b£®BŗĶD»„ĪŖĶ¬ĻµĪļ
c£®DµÄ·Šµć±ČĶ¬Ģ¼Ō×ÓŹżµÄĶéĢžøß
£Ø7£©FµÄŗĖ“Ź²ÕńĒāĘ×ĻŌŹ¾Ö»ÓŠŅ»×é·å£¬¢ŻµÄ»Æѧ·½³ĢŹ½ĪŖ_________________________”£

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2016-2017ѧğŗÓ±±Ź”×æŌ½ĮŖĆĖø߶žÉĻµŚŅ»“ĪŌĀæ¼»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
£Ø1£©ŅŃÖŖ2mol H2ĶźČ«Č¼ÉÕÉś³ÉŅŗĢ¬Ė®·Å³ö572 kJČČĮ攣Š“³öH2Č¼ÉÕČȵÄČČ»Æѧ·“Ó¦·½³ĢŹ½£ŗ_____________________”£
£Ø2£©ŌŚ25 ”ę”¢101 kPaĻĀ£¬1 g CH4£Øg£©ĶźČ«Č¼ÉÕÉś³ÉCO2ŗĶŅŗĢ¬H2O£¬·Å³ö55 kJµÄČČĮ棬Š“³öøĆ·“Ó¦µÄČČ»Æѧ·½³ĢŹ½ _______________________ ”£
£Ø3£©ŅŃÖŖČČ»Æѧ·½³ĢŹ½£ŗ
¢Ł2C2H2£Øg£©+5O2£Øg£©=4CO2£Øg£©£«2H2O£Øl£© ¦¤H1£½”Ŗ2602.0kJ•mol-1
¢ŚC£Øs£©+O2£Øg£©=CO2£Øg£© ”÷H2£½”Ŗ393.5kJ•mol-1
¢ŪH2£Øg£©+ O2£Øg£©=H2O£Øl£© ”÷H3=”Ŗ285.8kJ”¤mol-1
O2£Øg£©=H2O£Øl£© ”÷H3=”Ŗ285.8kJ”¤mol-1
Ōņ·“Ó¦¢Ü2C£Øs£©+H2£Øg£©=C2H2£Øg£©µÄ”÷HĪŖ ____________ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2016-2017ѧğøŹĖąŹ”øßŅ»ÉĻ10ŌĀŌĀæ¼»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗŃ”ŌńĢā
ĻÖÓŠČż×éČÜŅŗ£ŗ¢ŁĘūÓĶŗĶĀČ»ÆÄĘČÜŅŗ ¢Ś39£„µÄŅŅ“¼ČÜŅŗ ¢ŪĀČ»ÆÄĘŗĶµ„ÖŹäåµÄĖ®ČÜŅŗ£¬·ÖĄėŅŌÉĻø÷»ģŗĻŅŗµÄÕżČ··½·ØŅĄ“ĪŹĒ( )”£
A £®·ÖŅŗ”¢ŻĶČ””¢ÕōĮó B£®ŻĶČ””¢ÕōĮ󔢷ÖŅŗ
£®·ÖŅŗ”¢ŻĶČ””¢ÕōĮó B£®ŻĶČ””¢ÕōĮ󔢷ÖŅŗ
C£®·ÖŅŗ”¢ÕōĮó”¢ŻĶ Č” D£®ÕōĮó”¢ŻĶČ””¢·ÖŅŗ
Č” D£®ÕōĮó”¢ŻĶČ””¢·ÖŅŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2017½ģ¹ć¶«Ź”øßČżÉĻµŚ¶ž“ĪŌĀæ¼»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
[»Æѧ”Ŗ”ŖŃ”ŠŽ3£ŗĪļÖŹ½į¹¹ÓėŠŌÖŹ]
Mn”¢Fe¾łĪŖµŚĖÄÖÜĘŚ¹ż¶ÉŌŖĖŲ£¬Į½ŌŖĖŲµÄ²æ·ÖµēĄėÄÜŹż¾ŻĮŠÓŚĻĀ±ķ£ŗ
ŌŖ ĖŲ | Mn | Fe | |
µēĄėÄÜ | I1 | 717[ | 759 |
I2 | 1509 | 1561 | |
I3 | 3248 | 2957 | |
»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£© MnŌŖĖŲ¼Ūµē×Ó²ćµÄµē×ÓÅŲ¼Ź½ĪŖ £¬±Č½ĻĮ½ŌŖĖŲµÄI2”¢I3æÉÖŖ£¬ĘųĢ¬Mn2£«ŌŁŹ§Č„Ņ»øöµē×Ó±ČĘųĢ¬Fe2£«ŌŁŹ§Č„Ņ»øöµē×ÓÄŃ”£¶Ō“Ė£¬ÄćµÄ½āŹĶŹĒ £»
£Ø2£© FeŌ×Ó»ņĄė×ÓĶāĪ§ÓŠ½Ļ¶ąÄÜĮæĻą½üµÄæÕ¹ģµĄ¶ųÄÜÓėŅ»Š©·Ö×Ó»ņĄė×ÓŠĪ³ÉÅäŗĻĪļ”£
¢Ł ÓėFeŌ×Ó»ņĄė×ÓŠĪ³ÉÅäŗĻĪļµÄ·Ö×Ó»ņĄė×ÓÓ¦¾ß±øµÄ½į¹¹ĢŲÕ÷ŹĒ £»
¢Ś ĮłĒčŗĻŃĒĢśĄė×Ó£ØFe(CN)64££©ÖŠµÄÅäĢåCN£ÖŠCŌ×ÓµÄŌӻƹģµĄĄąŠĶŹĒ £¬Š“³öŅ»ÖÖÓėCN£»„ĪŖµČµē×ÓĢåµÄµ„ÖŹ·Ö×ÓµÄĀ·Ņ×Ė¹½į¹¹Ź½ £»
£Ø3£© ČżĀČ»ÆĢś³£ĪĀĻĀĪŖ¹ĢĢ壬ČŪµć282”ę£¬·Šµć315”ę£¬ŌŚ300”ęŅŌÉĻŅ×Éż»Ŗ”£Ņ×ČÜÓŚĖ®£¬Ņ²Ņ×ČÜÓŚŅŅĆŃ”¢±ūĶŖµČÓŠ»śČܼĮ”£¾Ż“ĖÅŠ¶ĻČżĀČ»ÆĢś¾§ĢåĪŖ £»
£Ø4£© ½šŹōĢśµÄ¾§ĢåŌŚ²»Ķ¬ĪĀ¶ČĻĀÓŠĮ½Öֶѻż·½Ź½£¬¾§°ū·Ö±šČēÓŅĶ¼ĖłŹ¾”£ĆęŠÄĮ¢·½¾§°ūŗĶĢåŠÄĮ¢·½¾§°ūÖŠŹµ¼Źŗ¬ÓŠµÄFeŌ×ÓøöŹżÖ®±ČĪŖ ”£

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2017½ģ¹ć¶«Ź”øßČżÉĻµŚ¶ž“ĪŌĀæ¼»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗŃ”ŌńĢā
ĻĀĮŠŠšŹö¢ńŗĶ¢ņ¾łÕżČ·²¢ÓŠŅņ¹ū¹ŲĻµµÄŹĒ£Ø £©
Ń”Ļī | ŠšŹö¢ń | ŠšŹö¢ņ |
A | 1-¼ŗ“¼µÄ·Šµć±Č¼ŗĶéµÄ·Šµćøß | 1-¼ŗ“¼ŗĶ¼ŗĶéæÉĶعżÕōĮó³õ²½·ÖĄė |
B | Ōµē³Ųæɽ«»ÆѧÄÜ×Ŗ»ÆĪŖµēÄÜ | Ōµē³ŲŠčĶā½ÓµēŌ“²ÅÄܹ¤×÷ |
C | ŅŅ¶žĖįæÉÓėKMnO4ČÜŅŗ·¢Éś·“Ó¦ | ŅŅ¶žĖį¾ßÓŠĖįŠŌ |
D | NaŌŚCl2ÖŠČ¼ÉÕµÄÉś³ÉĪļŗ¬Ąė×Ó¼ü | NaCl¹ĢĢåæɵ¼µē |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2017½ģø£½ØŹ”ĖĵŲĮłŠ£øßČżÉĻµŚŅ»“ĪŌĀæ¼»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗŃ”ŌńĢā
½«×ćĮæCO2Öš½„ĶØČėKOHŗĶCa(OH)2µÄ»ģŗĻČÜŅŗÖŠ£¬Éś³ÉµÄĪļÖŹŅĄ“ĪŹĒ£ØĖ®³żĶā£©£Ø £©
A£®K2CO3”¢KHCO3”¢CaCO3 ”¢Ca(HCO3)2
ӢCa(HCO3)2
B£®CaCO3”¢K2CO3”¢KHCO3”¢Ca(HCO3)2
C£®K2CO3”¢CaCO3”¢KHCO3”¢Ca(HCO3)2
D£®K2CO3”¢CaCO3”¢KHCO3”¢Ca(HCO3)2
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2017½ģø£½ØŹ”ĖĵŲĮłŠ£øßČżÉĻµŚŅ»“ĪŌĀæ¼»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗŃ”ŌńĢā
ijČÜŅŗæÉÄÜ“ęŌŚNa+”¢Ba2+”¢Mg2+”¢Fe3+”¢Br£”¢CO32£”¢Cl-µČĄė×Ó£¬Ä³Ń§Éś½ųŠŠĻĀĮŠŹµŃé£ŗȔɣĮæŌČÜŅŗ£¬²āµĆČÜŅŗ³ŹĒæ¼īŠŌ£¬ŌŚĘäÖŠµĪ¼Ó×ćĮæĀČĖ®£¬ÓŠĪŽÉ«ĪŽĪ¶ĘųĢå²śÉś£¬ČÜŅŗČŌĪŖĪŽÉ«”£¹ŲÓŚøĆŌČÜŅŗµÄĶĘ²āÕżČ·µÄŹĒ£Ø £©
A£®ČÜŅŗÖŠæÉÄÜ“ęŌŚMg2+ŗĶFe3+ B£®ČÜŅŗÖŠæÉÄÜ“ęŌŚBr£ŗĶCl-
C£®ČÜŅŗæÉÄÜ“ęŌŚBa2£«ŗĶCl- D£®ČÜŅŗÖŠŅ»¶Ø“ęŌŚNa+ŗĶCO32£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2016-2017ѧğŌĘÄĻŹ”Ēś¾øŹŠø߶žÉĻµŚŅ»“ĪŌĀæ¼»Æѧ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗŃ”ŌńĢā
ŅŃÖŖ»Æѧ·“Ó¦2C(s)+O2(g)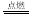 2CO(g)£»2CO(g) + O2(g)
2CO(g)£»2CO(g) + O2(g)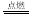 2CO2(g)¶¼ŹĒ·ÅČČ·“Ó¦”£¾Ż“ĖĶʶĻ£¬ĻĀĮŠĖµ·Ø²»ÕżČ·µÄŹĒ£ØĻąĶ¬Ģõ¼žĻĀ£©£Ø £©
2CO2(g)¶¼ŹĒ·ÅČČ·“Ó¦”£¾Ż“ĖĶʶĻ£¬ĻĀĮŠĖµ·Ø²»ÕżČ·µÄŹĒ£ØĻąĶ¬Ģõ¼žĻĀ£©£Ø £©
A£®56gCOŗĶ32gO2Ėł¾ßÓŠµÄ×ÜÄÜĮæ“óÓŚ88gCO2Ėł¾ßÓŠµÄ×ÜÄÜĮæ
B£®12gCĖł¾ßÓŠµÄÄÜĮæŅ»¶ØøßÓŚ28gCOĖł¾ßÓŠµÄÄÜĮæ
C£®12gCŗĶ32gO2Ėł¾ßÓŠµÄ×ÜÄÜĮæ“óÓŚ44gCO2Ėł¾ßÓŠµÄ×ÜÄÜĮæ
D£®½«Į½·ŻÖŹĮæĻąµČµÄĢ¼Č¼ÉÕ£¬Éś³ÉCO2µÄ·“Ó¦±ČÉś³ÉCOµÄ·“Ó¦·Å³öµÄČČĮæ¶ą
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2016-2017ѧğ¹ć¶«Ź”øßŅ»ÉĻµŚŅ»“Ī“óæ¼»Æѧ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗŃ”ŌńĢā
ĻĀĮŠŹµŃé²Ł×÷ÖŠ“ķĪóµÄŹĒ( )
A£®·ÖŅŗŹ±£¬·ÖŅŗĀ©¶·ĻĀ²ćŅŗĢå“ÓĻĀæŚ·Å³ö£¬ÉĻ²ćŅŗĢå“ÓÉĻæŚµ¹³ö
B£®ÕōĮóŹ±£¬Ó¦Ź¹ĪĀ¶Č¼ĘĖ®ŅųĒņææ½üÕōĮóÉÕĘæÖ§¹ÜæŚ“¦
C£®Õō·¢½į¾§Ź±Ó¦½«ČÜŅŗÕōøÉ
D£®³ĘĮæŹ±£¬³ĘĮæĪļ·ÅŌŚ³ĘĮæÖ½ÉĻÖĆÓŚĶŠÅĢĢģĘ½µÄ×óÅĢ£¬ķĄĀė·ÅŌŚĶŠÅĢĢģĘ½µÄÓŅÅĢÖŠ
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com