

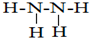 £»øĆŌŖĖŲ»¹æÉÓėŌŖĖŲ¢ŁŠĪ³É10µē×ÓµÄĘųĢå·Ö×ÓY£¬½«¹żĮæµÄYĘųĢåĶØČėŹ¢ÓŠŗ¬¢āŌŖĖŲµÄĮņĖįŃĪČÜŅŗÖŠ£¬·“Ó¦¹ż³ĢÖŠµÄŹµŃéĻÖĻóĪŖĻČ²śÉśĄ¶É«³Įµķ£¬Č»ŗó³ĮµķČܽā£¬±ä³ÉÉīĄ¶É«ČÜŅŗ£®
£»øĆŌŖĖŲ»¹æÉÓėŌŖĖŲ¢ŁŠĪ³É10µē×ÓµÄĘųĢå·Ö×ÓY£¬½«¹żĮæµÄYĘųĢåĶØČėŹ¢ÓŠŗ¬¢āŌŖĖŲµÄĮņĖįŃĪČÜŅŗÖŠ£¬·“Ó¦¹ż³ĢÖŠµÄŹµŃéĻÖĻóĪŖĻČ²śÉśĄ¶É«³Įµķ£¬Č»ŗó³ĮµķČܽā£¬±ä³ÉÉīĄ¶É«ČÜŅŗ£®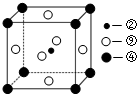
·ÖĪö ÓÉŌŖĖŲÖÜĘŚ±ķæÉŅŌµĆ³öŌŖĖŲ¢ŁĪŖH£¬¢ŚĪŖC£¬¢ŪĪŖO£¬¢ÜĪŖMg£¬¢ŻĪŖMn£¬¢ŽĪŖS£¬¢ßĪŖCl£¬¢ąĪŖCa£¬¢įĪŖNi£¬¢āĪŖCu£»
£Ø1£©¢Ś”¢¢Ü”¢¢į·Ö±šĪ»ÓŚ¾§°ūµÄĢåŠÄ”¢¶„µć”¢ĆęŠÄ£¬CŌ×ÓøöŹżĪŖ1£¬MgŌ×ÓøöŹżĪŖ8”Į$\frac{1}{8}$=1£¬NiŌ×ÓøöŹżĪŖ6”Į$\frac{1}{2}$=3£»
£Ø2£©ŌŖĖŲµÄĢŲÕ÷µē×ÓÅŲ¼Ź½ĪŖnsnnpn+1£¬Ōņn=2£¬¹ŹøĆŌŖĖŲµÄĢŲÕ÷µē×ÓÅŲ¼Ź½ĪŖ2s22p3£¬ĪŖNŌ×Ó£¬ŌŖĖŲÓėŌŖĖŲ¢ŁŠĪ³ÉµÄ18µē×ÓµÄX·Ö×ÓĪŖN2H4£»ŌŖĖŲ¢ŁĪŖHŌŖĖŲ£¬ÓėXŠĪ³ÉµÄĪļÖŹĪŖNH3£¬ÓėĮņĖįĶČÜŅŗĻČÉś³ÉĄ¶É«³Įµķ£¬ŗóŠĪ³ÉĀēŗĻĪļ£»
£Ø3£©·Ē½šŹōŠŌŌ½Ē棬µēøŗŠŌŌ½“ó£»
£Ø4£©Cuµ„ÖŹµÄ¾§Ģå¶Ń»ż·½Ź½ĪŖĆęŠÄĮ¢·½Ćܶѻż£¬ĄūÓĆ¾łĢƷؼĘĖć¾§°ūÖŠCuŌ×ÓŹżÄ棬ÉčCuŌ×Ó°ė¾¶ĪŖr£¬Ōņ¾§°ūĄā³¤ĪŖ4r”Į$\frac{\sqrt{2}}{2}$=2$\sqrt{2}$r£¬æÕ¼äĄūÓĆĀŹ=$\frac{¾§°ūÖŠŌ×Ó×ÜĢå»ż}{¾§°ūĢå»ż}$”Į100%£®
½ā“š ½ā£ŗÓÉŌŖĖŲÖÜĘŚ±ķæÉŅŌµĆ³öŌŖĖŲ¢ŁĪŖH£¬¢ŚĪŖC£¬¢ŪĪŖO£¬¢ÜĪŖMg£¬¢ŻĪŖMn£¬¢ŽĪŖS£¬¢ßĪŖCl£¬¢ąĪŖCa£¬¢įĪŖNi£¬¢āĪŖCu£»
£Ø1£©¢Ś”¢¢Ü”¢¢į·Ö±šĪ»ÓŚ¾§°ūµÄĢåŠÄ”¢¶„µć”¢ĆęŠÄ£¬CŌ×ÓøöŹżĪŖ1£¬MgŌ×ÓøöŹżĪŖ8”Į$\frac{1}{8}$=1£¬NiŌ×ÓøöŹżĪŖ6”Į$\frac{1}{2}$=3£¬»ÆѧŹ½ĪŖMgNi3C£¬¹Ź“š°øĪŖ£ŗMgNi3C£»
£Ø2£©ŌŖĖŲµÄĢŲÕ÷µē×ÓÅŲ¼Ź½ĪŖnsnnpn+1£¬Ōņn=2£¬¹ŹøĆŌŖĖŲµÄĢŲÕ÷µē×ÓÅŲ¼Ź½ĪŖ2s22p3£¬ĪŖNŌ×Ó£¬ŌŖĖŲÓėŌŖĖŲ¢ŁŠĪ³ÉµÄ18µē×ÓµÄX·Ö×ÓĪŖN2H4£¬Ęä½į¹¹Ź½ĪŖ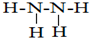 £»ŌŖĖŲ¢ŁĪŖHŌŖĖŲ£¬ÓėXŠĪ³ÉµÄĪļÖŹĪŖNH3£¬ÓėĮņĖįĶČÜŅŗĻČÉś³ÉĄ¶É«³Įµķ£¬ŗóŠĪ³ÉĀēŗĻĪļ£¬Ōņ¹Ū²ģµ½µÄĻÖĻóĪŖĻČ²śÉśĄ¶É«³Įµķ£¬Č»ŗó³ĮµķČܽā£¬±ä³ÉÉīĄ¶É«ČÜŅŗ£¬
£»ŌŖĖŲ¢ŁĪŖHŌŖĖŲ£¬ÓėXŠĪ³ÉµÄĪļÖŹĪŖNH3£¬ÓėĮņĖįĶČÜŅŗĻČÉś³ÉĄ¶É«³Įµķ£¬ŗóŠĪ³ÉĀēŗĻĪļ£¬Ōņ¹Ū²ģµ½µÄĻÖĻóĪŖĻČ²śÉśĄ¶É«³Įµķ£¬Č»ŗó³ĮµķČܽā£¬±ä³ÉÉīĄ¶É«ČÜŅŗ£¬
¹Ź“š°øĪŖ£ŗ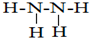 £»ĻČ²śÉśĄ¶É«³Įµķ£¬Č»ŗó³ĮµķČܽā£¬±ä³ÉÉīĄ¶É«ČÜŅŗ£»
£»ĻČ²śÉśĄ¶É«³Įµķ£¬Č»ŗó³ĮµķČܽā£¬±ä³ÉÉīĄ¶É«ČÜŅŗ£»
£Ø3£©·Ē½šŹōŠŌŌ½Ē棬µēøŗŠŌŌ½“ó£¬ŌņµēøŗŠŌ“ÓŠ”µ½“óĖ³ŠņŹĒCa£¼Al£¼S£¼Cl£¼O£¬¹Ź“š°øĪŖ£ŗCa£¼Al£¼S£¼Cl£¼O£»
£Ø4£©Cuµ„ÖŹµÄ¾§Ģå¶Ń»ż·½Ź½ĪŖĆęŠÄĮ¢·½Ćܶѻż£¬¾§°ūÖŠCuŌ×ÓŹżÄæĪŖ8”Į$\frac{1}{8}$+6”Į$\frac{1}{2}$=4£¬ÉčCuŌ×Ó°ė¾¶ĪŖr£¬Ōņ¾§°ūÖŠCuŌ×Ó×ÜĢå»żĪŖ4”Į$\frac{4}{3}$¦Šr3£¬ÉčCuŌ×Ó°ė¾¶ĪŖr£¬Ōņ¾§°ūĄā³¤ĪŖ4r”Į$\frac{\sqrt{2}}{2}$=2$\sqrt{2}$r£¬¾§°ūĢå»żĪŖ£Ø2$\sqrt{2}$r£©3=16$\sqrt{2}$r3£¬æÕ¼äĄūÓĆĀŹ=$\frac{\frac{16}{3}¦Š{r}^{3}}{16\sqrt{2}{r}^{3}}$”Į100%=74%£¬
¹Ź“š°øĪŖ£ŗĆęŠÄĮ¢·½×īĆܶѻż£»74%£®
µćĘĄ ±¾Ģā×ŪŗĻæ¼²éĪļÖŹ½į¹¹ÓėŠŌÖŹ£¬ĪŖøßĘµæ¼µć£¬Éę¼°ŌŖĖŲÖÜĘŚ±ķ”¢Ēā¼ü”¢¾§°ū½į¹¹Óė¼ĘĖć£¬ŹĒ¶Ōѧɜ×ŪŗĻÄÜĮ¦µÄ漲飬£Ø4£©ĪŖ½ā“šµÄÄŃµć£¬ŠčŅŖѧɜ¾ß±øŅ»¶ØµÄæÕ¼äĻėĻóÓėŹżŃ§¼ĘĖćÄÜĮ¦£¬ĢāÄæÄѶČÖŠµČ£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ¢Ł¢Ś¢Ū | B£® | ¢Ż | C£® | ¢Ū¢Ż | D£® | ¢Ü¢Ż |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| X | |||
| Y | X | R | |
| W |
| A£® | ĪåÖÖŌŖĖŲæÉÄܶ¼ŹĒ½šŹōŌŖĖŲ | |
| B£® | ĪåÖÖŌŖĖŲµÄŌ×Ó×īĶā²ćµē×ÓŹżŅ»¶Ø¶¼“óÓŚ2 | |
| C£® | XµÄĒā»ÆĪļµÄ·ŠµćŅ»¶Ø±ČZµÄĒā»ÆĪļµÄ·Šµćøß | |
| D£® | RµÄ×īøß¼ŪŃõ»ÆĪļ¶ŌÓ¦µÄĖ®»ÆĪļŅ»¶ØŹĒĒæĖį |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | MgCl2 | B£® | KNO3 | C£® | NaCl | D£® | £ØNH4£©2SO4 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ¼õŃ¹ | B£® | ½µĪĀ | C£® | ¼ÓŃ¹ | D£® | Ōö“óBµÄÅØ¶Č |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
 XÓėĒāŌŖĖŲŠĪ³ÉµÄ»ÆŗĻĪļµÄµē×ÓŹ½ŹĒ[H£ŗ]-Ca2+[£ŗH]-£®
XÓėĒāŌŖĖŲŠĪ³ÉµÄ»ÆŗĻĪļµÄµē×ÓŹ½ŹĒ[H£ŗ]-Ca2+[£ŗH]-£®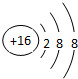 £»DÓėEÄÜŠĪ³ÉŅ»ÖÖ·Ö×Ó£¬øĆ·Ö×ӵĽį¹¹Ź½ĪŖS=C=S£»DĖłŌŚ×åŌŖĖŲµÄĒā»ÆĪļÖŠ£¬·Šµć×īµĶµÄŹĒĮņ»ÆĒā£ØĢīĆū³Ę£©£»XÓėEŠĪ³ÉµÄĄė×Ó»ÆŗĻĪļ XE2ÖŠÓŠ£ØĢī”°ÓŠ”±”°ĪŽ”±£©¹²¼Ū¼ü£®
£»DÓėEÄÜŠĪ³ÉŅ»ÖÖ·Ö×Ó£¬øĆ·Ö×ӵĽį¹¹Ź½ĪŖS=C=S£»DĖłŌŚ×åŌŖĖŲµÄĒā»ÆĪļÖŠ£¬·Šµć×īµĶµÄŹĒĮņ»ÆĒā£ØĢīĆū³Ę£©£»XÓėEŠĪ³ÉµÄĄė×Ó»ÆŗĻĪļ XE2ÖŠÓŠ£ØĢī”°ÓŠ”±”°ĪŽ”±£©¹²¼Ū¼ü£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ĻąĶ¬ĪļÖŹµÄĮæÅØ¶ČµÄFeI2ČÜŅŗÓėBr2Ė®ČÜŅŗµČĢå»ż»ģŗĻ£ŗ2Fe2++2I-+2Br2ØT2Fe3++I2+4Br- | |
| B£® | ĻņBa£ØOH£©2ČÜŅŗÖŠ¼ÓČė¹żĮæNH4HSO4ČÜŅŗ£ŗNH4++Ba2++2OH-+H++SO42-ØTBaSO4”ż+NH3•H2O+H2O | |
| C£® | ĻņĘÆ°×·ŪČÜŅŗÖŠĶØČė¹żĮæµÄSO2£ŗCa2++2ClO-+SO2+H2OØTCaSO3”ż+2HClO | |
| D£® | ĖÄŃõ»ÆČżĢśČÜÓŚĒāµāĖįČÜŅŗÖŠ£ŗFe3O4+8H++2I-ØT3Fe2++I2+4H2O |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
| CH4 £Øg£© | H2O £Øg£© | CO £Øg£© | H2 £Øg£© |
| 3.0mol•L-1 | 8.5mol•L-1 | 2.0mol•L-1 | 2.0mol•L-1 |
| ŹµŃéŠņŗÅ | ĪĀ¶Č/”ę | Ń¹Ēæ/kPa | V£ØCH4£©/mol•L-1•s-1 | V£ØH2O£©/mol•L-1•s-1 |
| 1 | 360 | P1 | 0.100 | 0.100 |
| 2 | 480 | 101 | 0.120 | 0.120 |
| 3 | 360 | P2 | 0.080 | 0.080 |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com