
84����Һ������Чɱ�����H1N1����,ijͬѧ������һƿijƷ�ơ�84����Һ��,������������Ϻ�����Һ��װ˵���õ�������Ϣ:
��84����Һ��:��25% NaClO��1 000 mL���ܶ�1.19 g��cm-3,ϡ��100��(�����)��ʹ�á�
�����������Ϣ�����֪ʶ�ش���������:
(1)�á�84����Һ�������ʵ���Ũ��Ϊ��������mol��L-1��
(2)��ͬѧȡ100 mL��Ʒ�ơ�84����Һ��ϡ�ͺ���������,ϡ�ͺ����Һ��c(Na+)����������mol��L-1(����ϡ�ͺ���Һ�ܶ�Ϊ1.0 g��cm-3)��
(3)��ͬѧ���ĸ�Ʒ�ơ�84����Һ�����䷽,����NaClO��������480 mL��25% NaClO������Һ������˵����ȷ��������������
A.ѡ��480 mL������ƿ
B.����ƿ������ˮϴ����,Ӧ��ɲ���������Һ����
C.���ù������ƷNaClO�����ƿ��ܵ��½��ƫ��
D.��Ҫ������NaClO��������Ϊ143 g
��������(1)����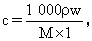
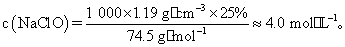
(2)4.0 mol��L-1��NaClO��Һϡ��100����,��Ũ��Ϊ0.04 mol��L-1,��c(Na+)=0.04 mol��L-1��
(3)ѡ��A,ʵ����û��480 mL������ƿ,Ӧѡ��500 mL������ƿ;ѡ��B,���ƹ�������Ҫ����ˮ,���Ծ�ϴ�Ӹɾ�������ƿ���غ�ɺ���ʹ��;ѡ��C,����NaClO�����տ����е�H2O��CO2������,������ƷNaClO���ܲ��ֱ��ʵ���NaClO����,���Ƶ���Һ�����ʵ����ʵ�����С,���ƫ��;ѡ��D,ѡȡ500 mL������ƿ��������,���밴500 mL��Һ�����������ʵ���,������ҪNaClO������Ϊ
0.5 L��4.0 mol��L-1��74.5 g��mol-1=149 g��
��:(1)4.0 (2)0.04 (3)C



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������������Ч���ٶ���������ŷš�ʵ�����÷�ú��(��Ҫ��Al2O3��SiO2��)�Ʊ���ʽ������[Al2(SO4)x(OH)6��2x]��Һ�����������������о���
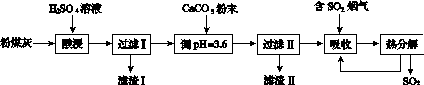
(1)���ʱ��Ӧ�Ļ�ѧ����ʽΪ____________________�����������Ҫ�ɷ�Ϊ________(�ѧʽ)��
(2)��CaCO3������Һ��pH��3.6����Ŀ�����к���Һ�е��ᣬ��ʹAl2(SO4)3ת��ΪAl2(SO4)x(OH)6��2x�����������Ҫ�ɷ�Ϊ________(�ѧʽ)������Һ��pHƫ�ߣ����ᵼ����Һ����Ԫ�صĺ������ͣ���ԭ����__________________________(�����ӷ���ʽ��ʾ)��
(3)���������о���ȫ�ȷֽ�ų���SO2������С�����յ�SO2��������Ҫԭ����________________________��������SO2ǰ����Һ��ȣ��ȷֽ��ѭ�����õ���Һ��pH��________(�������С�����䡱)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������Ϊ0.052 ��5.2%����NaOH��Һ1L���ܶ�Ϊ1.06g/cm3������Pt�缫���е�⣬����Һ��NaOH�����������ı���0.010��1.0%��ʱ��ֹͣ��⣬���ʱ��Һ��Ӧ���ϵĹ�ϵ�ǣ���
NaOH���������� �����������ʵ�����/g �����������ʵ�����/g
A 0.062��6.2%�� 19 152
B 0.062��6.2%�� 152 19
C 0.042��4.2%�� 1.2 9.4
D 0.042��4.2%�� 9.4 1.2
A�� A B�� B C�� C D�� D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ж����������л�ѧ��Ӧԭ���ķ�����ȷ���� (����)��
A�������������漰��������ѧ��Ӧ��ԭ�ϵIJ�ͬ����ȫ���Ƿ�������ԭ��
Ӧ
B�������������漰��������ѧ��Ӧ���Ƿ��ȷ�Ӧ
C�������������漰��������ѧ��Ӧ����Ҫʹ�ô���
D�������������漰��������ѧ��Ӧ����Ҫ�ڽϸ��¶������½���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����UNC��ѧ����Thomas J.Meyer���з��˻����Ѻá���ȫ�͵ġ���ɫ������ըҩ,����һ�ֿɱ�ʾΪNa2R,��������ˮ�п���ʧȥ����,��ը�����Σ���Բ������֪10 mL Na2R��Һ��Na+����ĿΪN,��Na2R��Һ�����ʵ���Ũ��Ϊ��(����)
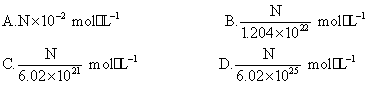
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���в���������,�������ʵ������Ե���(����)
A.��������ˮ B.�����ȥ����
C.�������ڲ;����� D.ʳ����ϴˮ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ڵؿ�����Ҫ��NaIO3����ʽ����,�ں�ˮ����Ҫ��I-����ʽ����,��������֮������ͼ��ʾ��ϵ,����ͼʾת����ϵ�Ʋ�����˵������ȷ���ǡ�(����)
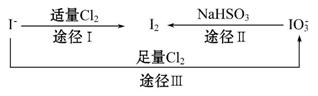
A.����KI-������ֽ��ʳ����ӵ������Ƿ��е�
B.����Cl2��ʹʪ���KI-������ֽ���ԭ�������5Cl2+I2+6H2O====2HIO3+
10HCl
C.��ͼ��֪�����Ե�ǿ��˳��ΪCl2>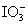 >I2
>I2
D.;������������1 mol I2,��Ӧ��ת�Ƶĵ�����Ϊ10NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪��ӦmX��g��+nY��g��⇌qZ��g���ġ�H��0��m+n��q���ں����ܱ������з�Ӧ�ﵽƽ��ʱ������˵����ȷ���ǣ�������
| �� | A�� | ͨ��ϡ������ʹѹǿ����ƽ�⽫�����ƶ� |
| �� | B�� | X������Ӧ������Y���淴Ӧ���ʵ� |
| �� | C�� | �����¶ȣ���������ƽ����Է���������С |
| �� | D�� | ����X�����ʵ�����Y��ת���ʽ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���з�Ӧ��������ȥ��Ӧ���ǣ���
A�� CH3CH2OH CH2=CH2��+H2O
CH2=CH2��+H2O
B�� CH3CHBrCH3+NaOH CH3CH=CH2��+NaBr+H2O
CH3CH=CH2��+NaBr+H2O
C��  +2NaOH
+2NaOH
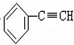 +2NaCl+2H2O
+2NaCl+2H2O
D�� 2CH3OH CH3﹣O﹣CH3+H2O
CH3﹣O﹣CH3+H2O
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com