
��ͼ�ش��������⣺
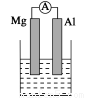
��1)���ձ�����ҺΪϡ���ᣬ��۲쵽��������______________________
������ӦʽΪ��_________________________________________________��
��2�����ձ�����ҺΪ����������Һ����Ϊ________(��Mg��Al)���ܷ�Ӧ��ѧ����ʽΪ
______________________________________________________________��
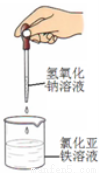
����Al��Cu��Ũ�������ԭ��أ��������ĵ缫��ӦʽΪ
��1���״���һ�ֿ�������Դ�����п�����Ӧ�õĹ���ǰ������ҵ��һ��ɲ������·�Ӧ���ϳɼ״��� CO(g)��2H2(g)  CH3OH(g)��
CH3OH(g)��
�����÷�Ӧ���ش��������⣺���и����У����ܹ�˵���÷�Ӧ�Ѵﵽƽ�����________(�����)��
a�����¡����������£���������ƽ����Է�����������
b��һ�������£�CH3OH�ֽ�����ʺ�CH3OH���ɵ��������
c��һ�������£�CO��H2��CH3OH��Ũ�ȱ��ֲ���
d��һ�������£���λʱ��������2 mol H2��ͬʱ����1 mol CH3OH
��2��2009��10�£��й���ѧԺ����Ӧ�û�ѧ�о����ڼ״�ȼ�ϵ�ؼ�����������ͻ�ƣ���װ�����Ժ�����ؼ�����ʽ��ѡ��״�ȼ�ϵ�صĹ���ԭ������ͼ��ʾ��
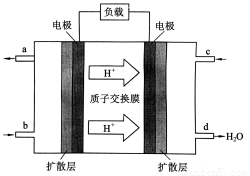
�ٸõ�ع���ʱ��b��ͨ�������Ϊ________________��
c��ͨ�������Ϊ________________��
�ڸõ�ظ����ĵ缫��ӦʽΪ��_______
�۹���һ��ʱ���6.4 g�״���ȫ��Ӧ����CO2ʱ��
��______________NA������ת�ơ�
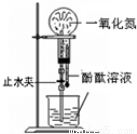
I��1��Mg���ܽ⣬AlƬ��������ð����ָ��ƫת��Mg-2e-=Mg2+
��2��Al��2Al+2NaOH+2H2O=2NaAlO2+3H2��
II��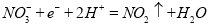
III.��1��d��2����CH3OH��O2�������CH3OH-6e-+H2O=CO2+6H+ ��1.2
��������
�����������1)���ձ�����ҺΪϡ���ᣬ��þΪ�������۲쵽��������Mg���ܽ⣬AlƬ��������ð����ָ��ƫת��������ӦʽΪMg-2e-=Mg2+����2�����ձ�����ҺΪ����������Һ����ΪAl���ܷ�Ӧ��ѧ����ʽΪ2Al+2NaOH+2H2O=2NaAlO2+3H2��������Al��Cu��Ũ�������ԭ��أ�Al��Ũ�����жۻ�����CuΪ�����������ĵ缫��ӦʽΪ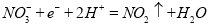 ����1��a�����¡����������£���������ƽ����Է����������䣬˵����������ʵ������ٸı䣬��Ӧ��ƽ�⣻b��һ�������£�CH3OH�ֽ�����ʺ�CH3OH���ɵ�������ȣ����淴Ӧ������ȣ���Ӧ��ƽ�⣻c��һ�������£�CO��H2��CH3OH��Ũ�ȱ��ֲ��䣬˵���Ѿ�ƽ�⣻d��һ�������£���λʱ��������2 mol H2��ͬʱ����1 mol CH3OH��Ϊͬһ��������˵���Ƿ�ƽ�⣻��ѡd����2�������״�ȼ�ϵ�ع���ʱ��bΪ����ͨ�������ΪCH3OH��c��ͨ�������ΪO2������������ĵ缫��ӦʽΪCH3OH-6e-+H2O=CO2+6H+������һ��ʱ���6.4 g�״���ȫ��Ӧ����CO2ʱ��ת��1.2mol���ӣ�����1.2NA������ת�ơ�
����1��a�����¡����������£���������ƽ����Է����������䣬˵����������ʵ������ٸı䣬��Ӧ��ƽ�⣻b��һ�������£�CH3OH�ֽ�����ʺ�CH3OH���ɵ�������ȣ����淴Ӧ������ȣ���Ӧ��ƽ�⣻c��һ�������£�CO��H2��CH3OH��Ũ�ȱ��ֲ��䣬˵���Ѿ�ƽ�⣻d��һ�������£���λʱ��������2 mol H2��ͬʱ����1 mol CH3OH��Ϊͬһ��������˵���Ƿ�ƽ�⣻��ѡd����2�������״�ȼ�ϵ�ع���ʱ��bΪ����ͨ�������ΪCH3OH��c��ͨ�������ΪO2������������ĵ缫��ӦʽΪCH3OH-6e-+H2O=CO2+6H+������һ��ʱ���6.4 g�״���ȫ��Ӧ����CO2ʱ��ת��1.2mol���ӣ�����1.2NA������ת�ơ�
���㣺����ԭ���ԭ�������֪ʶ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2013-2014����к����������ڶ���ģ�⿼�����ۻ�ѧ�Ծ��������棩 ���ͣ�ѡ����
��2 L�����ܱ������г���2 mol X��1mol Y������Ӧ��2X(g)+Y(g) 3Z(g) ��H��0����Ӧ���̳��������¶ȣ���û����ϵ��X������������¶ȵĹ�ϵ��ͼ��ʾ�������ƶ���ȷ����
3Z(g) ��H��0����Ӧ���̳��������¶ȣ���û����ϵ��X������������¶ȵĹ�ϵ��ͼ��ʾ�������ƶ���ȷ����

A�������¶ȣ�ƽ�ⳣ������
B��W��X������Ӧ���ʵ���M��X������Ӧ����
C��Q��ʱ��Y��ת�������
D��ƽ��ʱ����Z���ﵽ��ƽ��ʱZ�����������ԭƽ��ʱ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014����кӶ���������ģ�������ۻ�ѧ�Ծ��������棩 ���ͣ�ѡ����
����ҩƷ��װ�ú������������Ӧʵ�����
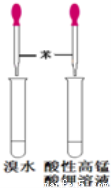
A B C D
A.��Ȫʵ�� B��ʵ������ȡ���ռ�����
C���Ʊ����������� D.��֤�����Ƿ���̼̼˫��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014����и���������¿����ۻ�ѧ�Ծ��������棩 ���ͣ������
��18�֣�B��һ����Ԫ��״�������˴Ź�������ֻ��һ���壻H�ĺ˴Ź���������3���壬�����֮��Ϊ2��2��1��I��һ�ֺϳ�����֬����Ҫԭ�ϡ���֪R���������й�����ת����ϵ����Ϣ���£�
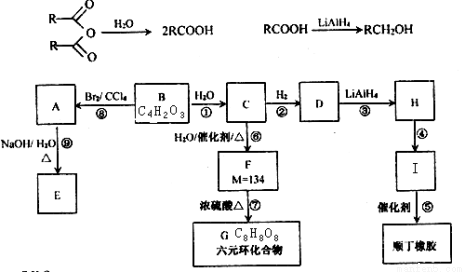
�밴Ҫ��ش��������⣺
��1��A�IJ�����������Ϊ _______________���۷�Ӧ����Ϊ____________________��
��2��B�Ľṹ��ʽΪ __________________��H��ϵͳ������Ӧ����Ϊ________________��
��3��д�����з�Ӧ�Ļ�ѧ����ʽ��
�� ____________________________________________________________________��
�� ____________________________________________________________________��
��4��д��˳���Ľṹ��ʽ_____________________________��˳�����Ըߣ��ͺ��Ժã����л������������͵��£��ϳ�·�����£�
���ȹ���(CH3)2SiCl2 ��������� (CH3)2Si(OH)2
��������� (CH3)2Si(OH)2 ����
����
��д�����۵Ļ�ѧ����ʽ__________________________________________________________��
��5���л���J��F��Ϊͬ���칹�壬�Ҿ�����ͬ�Ĺ������������Ŀ��д�����з���������J�Ľṹ��ʽ��������F����_________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014����и���������¿����ۻ�ѧ�Ծ��������棩 ���ͣ�ѡ����
��ѧ��������������ء�����˵������ȷ����
A����ǿ�����Ϳ�ͨ����ʳ���������������Ԫ��
B���ع������ڻ���һЩ�������к������ʶ�����ʳ�ã��ɼӹ��Ƴ�������ͣ�������ͳɷ����ʯ������ȡ�IJ��ͳɷֲ�ͬ
C������ý�屨����ij���е����ܼ��Ƕ����彡���к�������
D. ����β���е�CO��NO�ȶ������ͺͲ��͵IJ���ȫȼ�ղ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014����еڶ�ѧ�����м���һ��ѧ�Ծ��������棩 ���ͣ�ѡ����
Ԫ��R��X��T��Z��Q��Ԫ�����ڱ��е����λ�����±���ʾ�� ����R�����ڰ�����H2���һ��ϲ�������ը���������ж���ȷ����

A���ǽ����ԣ�Z<T<X B��R��Q�ĵ��������26
C����̬�⻯���ȶ��ԣ�R <T<Q D������������ˮ��������ԣ�T>Q
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014����еڶ�ѧ�����м���һ��ѧ�Ծ��������棩 ���ͣ�ѡ����
������,�� 4 mol A ����� 2 mol B ������ 2 L ���ܱ������л�ϲ���һ�������·������·�Ӧ��2A��g����B��g��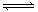 XC��g������ 2 s��Ӧ��ƽ��,��� C ��Ũ��Ϊ 0.6 mol��L��1 ��B�����ʵ���Ϊ1.4 mol,�������м���˵����
XC��g������ 2 s��Ӧ��ƽ��,��� C ��Ũ��Ϊ 0.6 mol��L��1 ��B�����ʵ���Ϊ1.4 mol,�������м���˵����
�������� A ��ʾ�ķ�Ӧ��ƽ������Ϊ 0.3 mol/(L��s)
�ڷ�Ӧǰ�����ڵ�ѹǿ��ƽ��������ڵ�ѹǿ֮��Ϊ1:1
�� 2 s ʱ����A��ת����Ϊ30�� �� X=2
������ȷ����
A���٢ۢ� B���٢� C���ڢ� D���ۢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014�������ѧ�����п��Ը߶���ѧ�Ծ��������棩 ���ͣ������
ͼ��A��G��Ϊ�л����������ͼ�е�ת����ϵ����Ӧ������ȥ�����ش��������⣺
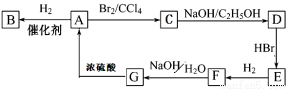
��1����״������A����Է�������Ϊ82�����к�̼87.80%������12.2%��B��һ�ȴ������һ�֣�B�Ľṹ��ʽΪ______________________________��
��2��M��B��һ��ͬ���칹�壬M��ʹ������Ȼ�̼��Һ��ɫ�����������е�̼ԭ�ӹ�ƽ�棬��M�Ľṹ��ʽΪ______________________________��
��3��д����C����D�Ļ�ѧ����ʽ______________________________��Ӧ������__________����G����A�Ļ�ѧ����ʽ______________________________��Ӧ������__________����G����F�Ļ�ѧ����ʽ______________________________��Ӧ������__________��G�����������Ļ�ѧ����ʽ______________________________��
��4��F�ĺ˴Ź�������ͼ��__________�����շ壬�����֮��Ϊ__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014������Ͽ���������ģ�Ծ����ۻ�ѧ�Ծ��������棩 ���ͣ�ʵ����
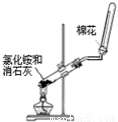
��18�֣��ס��������о���ѧϰС��Ϊ�ⶨ�������е�����ԭ�Ӹ����ȣ����������ʵ�����̣�
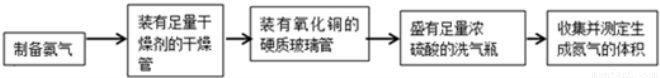
ʵ���У������Ƶõİ����ž�ϴ��ƿǰ����װ���еĿ�����������ϴ��ƿ�������ռ�װ �ã�������������ͭ����Ӧ��ɺ�ɫ����ͭת��Ϊ��ɫ��ͭ����ͼA��B��CΪ�ס�����С����ȡ����ʱ�����õ���װ�ã�DΪʢ��Ũ�����ϴ��ƿ��
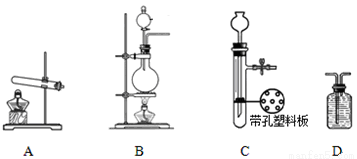
��С���ã���Ӧǰ����ͭ������m1 g������ͭ��Ӧ��ʣ����������m2g�����ɵ����ڱ�״���µ����V1L����С���ã�ϴ��ǰװ��D������m3g��ϴ����װ��D������Ϊm4g�����ɵ����ڱ�״���µ����V2L����ش��������⣺
��1�����Aװ�������ԵIJ�����____________________________________________________��
��2��ʵ���Ҽ��鰱���IJ�����������____________________________________��
��3���ס�����С��ѡ���˲�ͬ�ķ�����ȡ�������뽫ʵ��װ�õ���ĸ��ź��Ʊ��Ʊ�ԭ����д���±��Ŀո��С�
| ʵ��װ�� | ʵ��ҩƷ | �Ʊ�ԭ�� |
��С�� | A | �������ơ������ | ��Ӧ�Ļ�ѧ����ʽΪ____________ |
��С�� | _____ | Ũ��ˮ���������� | �û�ѧƽ��ԭ�������������Ƶ����ã�_______ |
��4����С�����������ݼ�����������е������ԭ�Ӹ���֮��Ϊ________________��
��5����С�����������ݼ�����������е������ԭ�Ӹ���֮������С������ֵ����ԭ����_______��Ϊ�ˣ���С����ԭ��ʵ��Ļ�����������һ��װ��ijҩ����ʵ������������ʵ�顣����ʵ��ǰ���ҩƷ�������仯�����ɵ�����������ó��˺�����ʵ��������ҩƷ��������________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com