
��0.02 mol Na��Ͷ�뵽ʢ��100 mLˮ��100 mL 1mol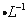 ���ᡢ100 mL 1mol
���ᡢ100 mL 1mol ����ͭ��Һ��X��Y��Z�����ձ��У������й�˵���������( )��
����ͭ��Һ��X��Y��Z�����ձ��У������й�˵���������( )��
A�������ձ���һ�����ᷢ�������ӷ�Ӧ�У�2Na+2H2O==2Na++2OH—+H2��
B�������ձ����ƾ���Һ���Ͼ��ҷ�Ӧ����ȶ��ԣ�X�ձ��еķ�Ӧƽ��Щ
C��Z�ձ���һ�����г������ɣ����������ǵ���ͭ
D�������ձ�����������������ʵ�����ͬ
��֪ʶ�㡿Ԫ�ػ����� C1
���𰸽�����A ������A��Na���Ⱥ������ᷴӦ�������ˮ��Ӧ����A����B��X�ձ���ֻ��ˮ���ʷ�Ӧ���ƽ������B��ȷ��C��Z�ձ���Na���Ⱥ�ˮ��Ӧ�������������ƻ������ͭ��Ӧ����������ͭ��������C��ȷ��D��X��Z�ձ��ж���2Na+2H2O==2Na++2OH—+H2����Y�ձ�����2Na+2HCl==2NaCl+H2����D��ȷ��
��˼·�㲦�����⿼����Na�����ʣ�ע��Na���ᷴӦ����η�Ӧ���Ѷ��еȡ�


 ���ſ����ϵ�д�
���ſ����ϵ�д� ���Ŀ����ϵ�д�
���Ŀ����ϵ�д� ������ӱ������ͯ������ϵ�д�
������ӱ������ͯ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�±���������֮��ͨ��һ����Ӧ������ʵ������ͼ��ʾת����ϵ����
| ѡ�� | X | Y | Z |
���ֵķ�Ӧ���� |
| A | Na2O2 | NaOH | NaCl | �� ������ˮ |
| B | AlCl3 | NaAlO2 | Al(OH)3 | �� ͨ��CO2 |
| C | NO | NO2 | HNO3 | �� ����ͭ�� |
| D | Cl2 | NaClO | HClO | �� ��Ũ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ס�����ͬѧ���ֱ���ɡ�����������Ӧ����ʵ�飮
I����ͬѧ�ķ���Ϊ��ȡһ���̶���Ľ����ƣ���ȥ�����㣩������ֽ����ú�ͣ�����ʯ�����ϣ��þƾ����ȣ������۳���״ʱ����ʢ�������ļ���ƿѸ�ٵ������Ƶ��Ϸ���װ����ͼI�����÷����IJ���֮���� ��Ԥ���ڿ����м��ȣ������������Ӱ������������ȼ�գ�ʵ������л����������Ⱦ �����ٴ�����㣩��
II����ͬѧ�����õ�װ����ͼ�ش��������⣺

��1����ͼ����װ����������ҩƷ��ʵ�鿪ʼ���Ƚ�Ũ���ἷ���Թܣ��Թ��з�����Ӧ�����ӷ���ʽΪ 2MnO4-+10Cl-+16H+=2Mn2++5Cl2��+8H2O����װ���г��� �����ȼ�ƾ��ƣ�
��2����ȼ�ƾ��ƺ������г��ֵ������� ���ۻ���ȼ�գ�������ɫ���棬�а��̣����ٴ�����㣩
��3����ͬѧ���������װ�ø�Ϊͼ����ʾװ�ã���������������������
����ͼ����ʾ����װ���ɸ���ܡ��齺�ܺ�50mL�ζ�����װ���ɣ��˴����õζ�����
��ʽ�����ʽ����ʽ�����ζ��ܣ�
��Ϊ��߲�����ȷ�ԣ�ͼ��װ���е�Һ����� ����NaCl��Һ���ռ������岢��ȴ�����º����������ǰӦ���еIJ����� .
�������ʼ����ʱ������ȷ��������ʱ�����ұߵζ���Һ�棬�ᵼ�������������� ���ƫ����ƫС������Ӱ�족����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
һ�������£���Na��O2��Ӧ��������1.5g����ˮ��������Һǡ���ܱ�80mLŨ��Ϊ
0.5 mol·L-1��HCl��Һ�кͣ����������ijɷ���
A. Na2O B. Na2O2 C. Na2O��Na2O2 D. Na2O2��NaO2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����Ƶ�˼ά�����ڻ�ѧѧϰ���о�����ʱ�����������ۣ�������ƵĽ�������Ҫ����ʵ���ļ��飬���ܾ�������ȷ������м������ƽ����У���������
������ˮ��Ӧ����NaOH��H2�����н�����ˮ��Ӧ�����ɼ��H2
����¶���ڿ�����һ��ʱ���ͻ����⣻���ʸ����õ��������ȶ������ڿ�����
�ۻ�����NaCl����ɫΪ��ɫ��Na2CO3����ɫҲΪ��ɫ
���ܶ�Ϊ1.1 g·cm��3���ܶ�Ϊ1.2 g·cm��3��NaCl��Һ�������ϣ�����NaCl��Һ���ܶȽ���1.1 g·cm��3��1.2 g·cm��3֮�䣬Na��K�Ͻ���۵�Ӧ����Na��K�۵�֮��
A���٢� B���٢� C���٢ڢۢ� D���٢ڢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��Ϣһ����ͬ�����ܡ����Ƚ������Թ��ɸ��ºϽ𡢵��ȺϽ𡢾��ܺϽ�ȣ����ں��ա�����������DZ��ȹ�ҵ���š�
��Ϣ�����Ȼ�����(CrO2Cl2)�Ǹ���һ�ֻ���������¸û������ǰ���ɫҺ�壬�۵�Ϊ-96.5�棬�е�Ϊ117�棬�ܺͱ�ͪ(CH3COCH3)�����Ȼ�̼��CS2���л��ܼ����ܡ�
��1����(24��Ԫ��)ԭ�ӵĻ�̬�����Ų�ʽΪ___________��
��2��CH3COCH3�����к���________���м�������____________���Ҽ���
��3����̬�Ȼ���������______________���壬��ͪ��̼ԭ�ӵ��ӻ���ʽΪ______________������̼����______________(����ԡ��Ǽ��ԡ�)���ӣ������к���____________(����ԡ��Ǽ��ԡ�)����
��4��K[Cr(C2O4)2(H2O)2]Ҳ�Ǹ���һ�ֻ�����û������������ӻ�������г������Ӽ������ۼ��⣬������____________����
��5���������ľ�����ͼ��ʾ��һ�������к���_______����ԭ�ӡ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵����ȷ����(����)
A��ǿ�������Һһ�������������Һ�ĵ�����ǿ
B��ǿ����ʵ�ϡ��Һ�в��������ʷ���
C��ǿ����ʶ������ӻ������������ʶ��ǹ��ۻ�����
D����ͬ���������ֻҪ���ʵ�����Ũ����ͬ������̶�Ҳ��ͬ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij�ռ���Ʒ���������������õ����ʣ�Ϊ�˲ⶨ�䴿�ȣ��������µζ�������
A����250 mL������ƿ�ж������250 mL�ռ���Һ
B������Һ����ȡ25 mL�ռ���Һ����ƿ�в��μ��μ���ָʾ��
C������ƽ��ȷ��ȡ�ռ���ƷW g�����ձ���������ˮ�ܽ�
D�������ʵ���Ũ��Ϊc�ı�������Һװ����ʽ�ζ��ܣ�����Һ�棬���¿�ʼ����ΪV1
E������ƿ�µ�һ�Ű�ֽ���ζ����յ㣬���¶���V2
�ش����и����⣺
��1����ȷ���������˳����______��______��______��D��______(������ĸ��д)��
(2)�ζ��ܵĶ���Ӧע��
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________��
��3��E������ƿ�µ�һ�Ű�ֽ��������
________________________________________________________________________
________________________________________________________________________��
D������Һ��Ӧ���ڵ�
________________________________________________________________________
__________�����첿��Ӧ__________________________________________________��
�ζ��յ�ʱ��ƿ����Һ��pHԼΪ_____________________________________��
�յ�ʱ��ɫ�仯��_______________________________________________________��
(6)����ʽ�ζ��ܲ��ñ�������ϴ����������������ȷ��ǰ���£���Բⶨ���(ָ�ռ�Ĵ���)�к�Ӱ�죿______(�ƫ�ߡ�����ƫ�͡����䡱)��
(7)���ռ���Ʒ���ȵļ���ʽΪ____________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ƿ����Ϊ����ʵ��ġ�����ƿ��������Ϊ��������ϲ����ܺ�������������ɸ��ֹ��ܵ�װ�á����и�ͼ���������������� ȫƿ���ǣ�������
ȫƿ���ǣ�������
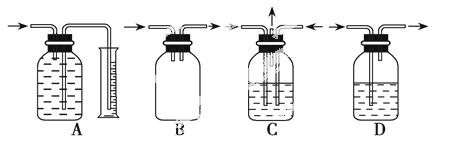
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com