
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 [��ѧ--���ʽṹ������
[��ѧ--���ʽṹ������ �鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2��ƫ���ᱵ��С�ͱ�ѹ������Ͳ���������ж���Ӧ�ã�ƫ���ᱵ�����о����Ľṹ����ͼ��ʾ�����Ļ�ѧʽ��
��2��ƫ���ᱵ��С�ͱ�ѹ������Ͳ���������ж���Ӧ�ã�ƫ���ᱵ�����о����Ľṹ����ͼ��ʾ�����Ļ�ѧʽ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ��ɽ��ʡ�����и���ģ���⣨һģ�����ۻ�ѧ�Ծ��������棩 ���ͣ������
�Ѻ��ѵĺϽ��ѱ��㷺���������Ѷ���ġ���������������豸���ɻ��Ⱥ��캽�ղ��ϣ�����Ϊ��δ������Ľ��������Իش��������⣺
��1������ ��
�� ����ԭ�ӣ����ǻ���Ϊ???? ���ѻ�̬ԭ�ӵĵ����Ų�ʽΪ?????????????????? ��
����ԭ�ӣ����ǻ���Ϊ???? ���ѻ�̬ԭ�ӵĵ����Ų�ʽΪ?????????????????? ��
��2��ƫ���ᱵ��С�ͱ�ѹ������Ͳ���������ж���Ӧ�á�ƫ���ᱵ�����о����Ľṹ��ͼ��ʾ�����Ļ�ѧʽ��?????????? ��
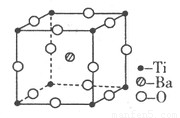
��3������TiO2��һ��Ӧ�ù㷺�Ĵ���������TiO2����һ��ʵ�����£�

������ķ����в�ȡsp2�ӻ���̼ԭ�Ӹ���Ϊ???????? �����������в�ȡsp3�ӻ���ԭ�ӵĵ�һ�������ɴ�С��˳��Ϊ?????????? ��
��4�����к�Ti3+��������ѧʽΪ[TiCl(H2O)5]Cl2��H2O��������[TiCl(H2O)5] 2+??? �к��еĻ�ѧ��������????? ����������������????? ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�츣��ʡ�����и߶���ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ������
��8�֣����Ѻ��ѵĺϽ��ѱ��㷺���������Ѷ���ġ���������������豸���ɻ��Ⱥ��캽�ղ��ϣ�����Ϊ��δ������Ľ��������Իش��������⣺
��1������ Ti��
Ti�� Ti����ԭ�ӣ����ǻ���Ϊ ��TiԪ����Ԫ�����ڱ��е�λ���ǵ�
���ڣ��� �壻�������Ų�TiԪ����Ԫ�����ڱ�����������
����s��p��d��ds��f����Ԫ��
Ti����ԭ�ӣ����ǻ���Ϊ ��TiԪ����Ԫ�����ڱ��е�λ���ǵ�
���ڣ��� �壻�������Ų�TiԪ����Ԫ�����ڱ�����������
����s��p��d��ds��f����Ԫ��
��2��ƫ���ᱵ��С�ͱ�ѹ������Ͳ���������ж���Ӧ�á�ƫ���ᱵΪ���Ӿ��壬�����Ľṹ����ͼ��ʾ�����Ļ�ѧʽ��

��3�����к�Ti3+��������ѧʽΪTiCl3��H2O��6����1mol����������ˮ������������������Һ�������������Ȼ���Ϊ1mol����֪����������λ��Ϊ6�������������λ���� ��
1mol���������������ᾧˮ���ʵ���Ϊ mol��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com