
 C3N6H6 + 6NH3 + 3CO2
C3N6H6 + 6NH3 + 3CO2
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

 �鷽���� ��
�鷽���� ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
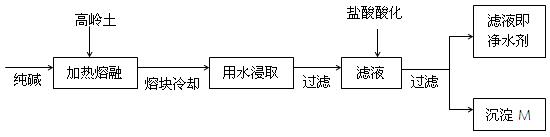
| A�������� | B�������� | C������������ | D���������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����ҵ���������ڽӴ���������������õ��������� |
| B����ҵ��ͨ����ⱥ���Ȼ�����Һ�Ʊ������� |
| C����ҵ�ð����������������˹��̵����� |
| D��������ͨ��������Ҫԭ����ʯ��ʯ��ʯӢ�ʹ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
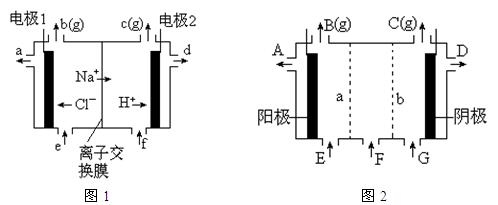
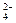 �����ϸߣ��������ӱ�ʽ����ȥSO
�����ϸߣ��������ӱ�ʽ����ȥSO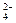 ���ñ��Լ������� �� ��
���ñ��Լ������� �� ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 2NH3(g) ��H����92.2kJ/mol���ϳɰ���ҵ��ԭ����N2�ɴӿ����з���õ���H2���ü����ڸ�������ˮ������Ӧ�Ƶá��ҹ��ϳɰ���ҵĿǰ����������Ϊ������������ý���¶ȣ�400��500�棬ѹǿ��30��50MPa��
2NH3(g) ��H����92.2kJ/mol���ϳɰ���ҵ��ԭ����N2�ɴӿ����з���õ���H2���ü����ڸ�������ˮ������Ӧ�Ƶá��ҹ��ϳɰ���ҵĿǰ����������Ϊ������������ý���¶ȣ�400��500�棬ѹǿ��30��50MPa��| �� �� | ȼ���ȣ�kJ��mol��1�� |
| H2(g) | ��285.8 |
| CO(g) | ��283.0 |
| CH4(g) | ��890.3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 2NH3(g) ��H=" -92.4" kJ/mol �ݴ˻ش��������⣺
2NH3(g) ��H=" -92.4" kJ/mol �ݴ˻ش��������⣺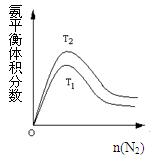
 H
H 2(g)+CO(g) ��ƽ�ⳣ��K1
2(g)+CO(g) ��ƽ�ⳣ��K1 H2(g)+CO2(g) ƽ�ⳣ��K2
H2(g)+CO2(g) ƽ�ⳣ��K2| ʵ����� | �¶�/�� | ��ʼ��/mol | ƽ����/mol | �ﵽƽ������ʱ��/min | ||
| H2O | CO | H2 | CO | |||
| 1 | 650 | 1 | 2 | 0.8 | 1.2 | 5 |
| 2 | 900 | 0.5 | 1 | 0.2 | 0.8 | 3 |
| 3 | T | a | b | c | d | t |
| A���������������¶�T<900�� | B���������������¶�T>900�� |
| C������һ�������� | D��ʹ�ø�Ч���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��ͨ��ú�ĸ�����Ի�ý�̿ |
| B��ú�к��б��ͼױ�������������ķ��������Ƿ������ |
| C��ͨ��ú�ĸ�����Ի�ôְ�ˮ |
| D��úҺ������Եõ��״� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com