
��ͬѧ��������װ����֤ľ̿��Ũ���ᷴӦ��ȫ������
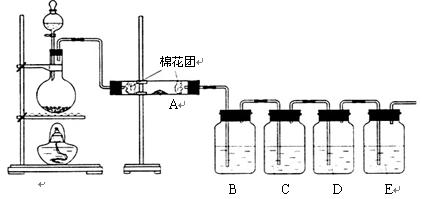
��1��д��ľ̿��Ũ���ᷴӦ�Ļ�ѧ����ʽ��
��2��A�м�����Լ��� ��B��D�м�����Լ�����Ʒ����Һ��D����ȷ��ʵ�������ǣ�
��3��ʵ��ʱ��C����������ǵ��е��۵ĵ�ˮ���۲쵽�������� ��
���ӷ���ʽΪ�� ��
����ͬѧֻ��B��C��D��Eװ����֤SO2��ijЩ���ʣ���ش��������⣺
��1��C�м�����Լ��� ��֤��SO2���������ԡ�
��2��D�м������Ե�KMnO4��Һ��֤��SO2���� �ԡ�
��3��E�м�����з�̪��NaOH��Һ��֤��SO2�� �����塣
 �żӾ���ϵ�д�
�żӾ���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ||
| �� |
| ||
| �� |
| 2016��6 |
| 2688��5 |
| 2016��6 |
| 2688��5 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡģ���� ���ͣ��ƶ���

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com