
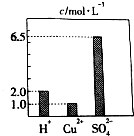
 ��0.2L��H2SO4��CuSO4��Al2��SO4��3��ɵĻ����Һ�У���������Ũ�ȴ�С��ͼ��ʾ����ش��������⣺
��0.2L��H2SO4��CuSO4��Al2��SO4��3��ɵĻ����Һ�У���������Ũ�ȴ�С��ͼ��ʾ����ش��������⣺���� ����ͼ���֪����Һ��c��H+��=2.0mol/L��c��Cu2+��=1.0mol/L��c��SO42-��=6.5mol/L��������Һ���Ϊ0.2L������Һ��n��H+��=CV=2.0mol/L��0.2L=0.4mol��n��Cu2+��=CV=1.0mol/L��0.2L=0.2mol��c��SO42-��=CV=6.5mol/L��0.2L=1.3mol���ɴ˷������
��� �⣺����ͼ���֪����Һ��c��H+��=2.0mol/L��c��Cu2+��=1.0mol/L��c��SO42-��=6.5mol/L��������Һ���Ϊ0.2L������Һ��n��H+��=CV=2.0mol/L��0.2L=0.4mol��n��Cu2+��=CV=1.0mol/L��0.2L=0.2mol��c��SO42-��=CV=6.5mol/L��0.2L=1.3mol��
��1��������Һ��H2SO4��CuSO4��Al2��SO4��3�����ʵ����ֱ�Ϊx��y��zmol��
������������Դ��H2SO4��֪��2x=0.4 ��
����ͭ����������CuSO4��֪��2y=0.2 ��
������������������߿�֪��x+y+3z=1.3 ��
��٢ڢۿɵ�x=0.2mol
y=0.2mol
z=0.3mol��Al3+�����ʵ���Ũ��Ϊ$\frac{0.3}{0.2}��2$=3mol/L��Al2��SO4��3�����ʵ���Ϊ0.3mol���ʴ�Ϊ��3mol/L��0.3mol��
��2����������Һ�м���������BaCl2��Һ����Ӧ�����ӷ���ʽΪBa2++SO42-=BaSO4�������������ʵ���������������ӵ����ʵ�����0.2+0.2+0.3��3=1.3mol�����Բ�������������Ϊ1.3mol��233g/mol=302.9g���ʴ�Ϊ��Ba2++SO42-=BaSO4����302.9g��
��3������û����Һ�м����������ۣ�������ͭ���ӷ�Ӧ��Ȼ���������ӷ�Ӧ����Һ�з�����Ӧ�����ӷ���ʽΪ��Fe+Cu2+=Cu+Fe2+��Fe+2H+=Fe2++H2��������0.2mol����������0.2mol������0.2mol��ͭ��������0.2mol���������Է�Ӧ����Һ��Fe2+��Ũ��Ϊ$\frac{��0.2+0.2��mol}{0.2L}$=2mol/L���ʴ�Ϊ��Fe+Cu2+=Cu+Fe2+��Fe+2H+=Fe2++H2����2mol/L��
���� ���⿼�����ʵ���Ũ�ȵļ��㣬���ط��������ͼ��������Ŀ��飬��Ŀ�ѶȲ���


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ʵ����� | B�������������� | ��Ӧ�������ܶȣ��ѻ���Ϊ��״����g/L�� |
| ��һ�� | 1.0 | 1.35 |
| �ڶ��� | 1.2 | 1.25 |
| ������ | 2.0 | 1.04 |
| ���Ĵ� | 2.2 | -- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
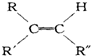 $��_{��Zn/H_{2}O}^{��O_{3}}$
$��_{��Zn/H_{2}O}^{��O_{3}}$ +
+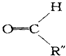 ��
�� ��C
��C ��E
��E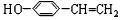 ��
��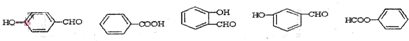 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��FeCl3��Һ��ʴͭ��·�壺Cu+2Fe3+�TCu2++2Fe2+ | |
| B�� | Na2O2��H2O��Ӧ�Ʊ�O2��Na2O2+H2O�T2Na++2OH-+O2�� | |
| C�� | ����������ˮ�Ʊ������Cl2+H2O�T2H++Cl-+ClO- | |
| D�� | ��Ũ�����ữ��KMnO4��Һ��H2O2��Ӧ��֤��H2O2���л�ԭ�ԣ�2MnO4-+6H++5H2O2�T2Mn2++5O2��+8H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com