Θ®2009?Ν…ΡΰΡΘΡβΘ©ΆΦ «“ΜΗω Β―ι “÷Τ»Γ¬»Τχ≤Δ“‘¬»ΤχΈΣ‘≠ΝœΫχ––ΧΊΕ®Ζ¥”ΠΒΡΉΑ÷ΟΘΚ
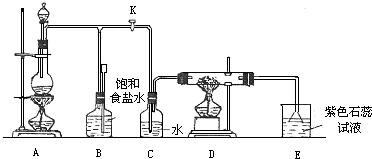
Θ®1Θ©A «¬»ΤχΖΔ…ζΉΑ÷ΟΘ§Τδ÷–Ζ¥”ΠΒΡΜ·―ßΖΫ≥ΧΈΣ
4HClΘ®≈®―ΈΥαΘ©+MnO
2MnCl
2+2H
2O+Cl
2Γϋ
4HClΘ®≈®―ΈΥαΘ©+MnO
2MnCl
2+2H
2O+Cl
2Γϋ
Θ°
Θ®2Θ© Β―ιΩΣ Φ ±Θ§œ»Βψ»ΦA¥ΠΒΡΨΤΨΪΒΤΘ§¥ρΩΣ–ΐ»ϊKΘ§»ΟCl
2≥δ¬ζ’ϊΗωΉΑ÷ΟΘ§‘ΌΒψ»ΦD¥ΠΨΤΨΪΒΤΘ§Ν§Ϋ”…œEΉΑ÷ΟΘ°Cl
2Ά®ΙΐCΤΩΚσ‘ΌΫχ»κDΘ°DΉΑ÷ΟΒΡ”≤÷ ≤ΘΝßΙήΡΎ Δ”–ΧΩΖέΘ§ΖΔ…ζ―θΜ·ΜΙ‘≠Ζ¥”ΠΘ§Τδ≤ζΈοΈΣCO
2ΚΆHClΘ° ‘–¥≥ωD÷–Ζ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΘΚ
2Cl
2+2H
2OΘ®ΤχΘ©+C
4HClΓϋ+CO
2Γϋ
2Cl
2+2H
2OΘ®ΤχΘ©+C
4HClΓϋ+CO
2Γϋ
Θ°ΉΑ÷ΟCΒΡΉς”Ο «
Έϋ ’Cl2÷–ΒΡHClΤχΧεΘ§ΧαΙ©D¥ΠΥυ–ηΥ°’τΤχ
Έϋ ’Cl2÷–ΒΡHClΤχΧεΘ§ΧαΙ©D¥ΠΥυ–ηΥ°’τΤχ
Θ°
Θ®3Θ©‘ΎE¥ΠΘ§Ήœ…Ϊ ·»ο ‘“ΚΒΡ―’…Ϊ”…Ήœ…Ϊ±δΈΣΚλ…ΪΘ§‘Ό±δΈΣΈό…ΪΘ§Τδ‘≠“ρ «
…ζ≥…ΒΡHClΤχΧε ΙΉœ…Ϊ ·»ο»ή“Κ±δΚλΘ§“ρΈ¥Ζ¥”ΠΆξΒΡCl2”κH2OΉς”Ο≤ζ…ζΒΡHClOΒΡΤ·ΑΉΉς”Ο ΙΚλ…Ϊœϊ ßΘ°
…ζ≥…ΒΡHClΤχΧε ΙΉœ…Ϊ ·»ο»ή“Κ±δΚλΘ§“ρΈ¥Ζ¥”ΠΆξΒΡCl2”κH2OΉς”Ο≤ζ…ζΒΡHClOΒΡΤ·ΑΉΉς”Ο ΙΚλ…Ϊœϊ ßΘ°
Θ°
Θ®4Θ©»τΫΪE¥Π…’±≠÷–»ή“ΚΗΡΈΣ≥Έ«ε ·Μ“Υ°Θ§Ζ¥”ΠΙΐ≥Χœ÷œσΈΣ
B
B
Θ°Θ®―ΓΧν±ξΚ≈Θ©
Θ®AΘ©”–ΑΉ…Ϊ≥ΝΒμ…ζ≥… Θ®BΘ©Έόœ÷œσ Θ®CΘ©œ»…ζ≥…ΑΉ…Ϊ≥ΝΒμΘ§ΕχΚσΑΉ…Ϊ≥ΝΒμœϊ ß
Θ®5Θ©D¥ΠΖ¥”ΠΆξ±œΚσΘ§ΙΊ±’–ΐ»ϊKΘ§“Τ»ΞΨΤΨΪΒΤΘ§ΒΪ”…”Ύ”ύ»»ΒΡΉς”ΟΘ§A¥Π»‘”–Cl
2≤ζ…ζΘ§¥Υ ±B÷–ΒΡœ÷œσ «
ΤΩ÷–“ΚΟφœ¬ΫΒΘ§≥ΛΨ±¬©ΕΖΡΎ“ΚΟφ…œ…ΐ
ΤΩ÷–“ΚΟφœ¬ΫΒΘ§≥ΛΨ±¬©ΕΖΡΎ“ΚΟφ…œ…ΐ
Θ§BΒΡΉς”Ο «
÷ϋ¥φ…ΌΝΩCl2
÷ϋ¥φ…ΌΝΩCl2
Θ°

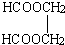 Θ©–¥≥ωΗς≤ΫΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΘ°Θ®Ω…≤ΜΉΔΟςΖ¥”ΠΧθΦΰΘ©
Θ©–¥≥ωΗς≤ΫΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΘ°Θ®Ω…≤ΜΉΔΟςΖ¥”ΠΧθΦΰΘ©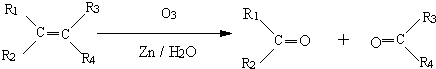 Θ®R1ΓΔR2ΓΔR3ΓΔR4ΓΔΩ…¥ζ±μΧΰΜυΘ§“≤Ω…¥ζ±μ«β‘≠Ή”Θ©
Θ®R1ΓΔR2ΓΔR3ΓΔR4ΓΔΩ…¥ζ±μΧΰΜυΘ§“≤Ω…¥ζ±μ«β‘≠Ή”Θ©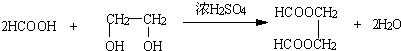
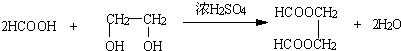
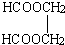 Θ§Ω…œ»÷Τ±ΗHCOOHΚΆHOCH2CH2OHΘ§Ω…“‘CH2CH=CH2ΈΣ‘≠ΝœΘ§―θΜ· «HCHOΚΆCH3CHOΘ§HCHO±Μ―θΜ·ΈΣHCOOHΘ§CH3CHO±ΜΜΙ‘≠ΈΣCH3CH2OHΘ§»ΜΚσCH3CH2OHΖΔ…ζœϊ»ΞΖ¥”Π…ζ≥…CH2=CH2Θ§CH2=CH2ΖΔ…ζΦ”≥…Ζ¥”Π…ζ≥…CH3BrCH2BrΘ§Υ°ΫβΩ……ζ≥…HOCH2CH2OHΘ§Εΰ’ΏΖΔ…ζθΞΜ·Ζ¥”ΠΩ……ζ≥…
Θ§Ω…œ»÷Τ±ΗHCOOHΚΆHOCH2CH2OHΘ§Ω…“‘CH2CH=CH2ΈΣ‘≠ΝœΘ§―θΜ· «HCHOΚΆCH3CHOΘ§HCHO±Μ―θΜ·ΈΣHCOOHΘ§CH3CHO±ΜΜΙ‘≠ΈΣCH3CH2OHΘ§»ΜΚσCH3CH2OHΖΔ…ζœϊ»ΞΖ¥”Π…ζ≥…CH2=CH2Θ§CH2=CH2ΖΔ…ζΦ”≥…Ζ¥”Π…ζ≥…CH3BrCH2BrΘ§Υ°ΫβΩ……ζ≥…HOCH2CH2OHΘ§Εΰ’ΏΖΔ…ζθΞΜ·Ζ¥”ΠΩ……ζ≥…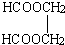 Θ°
Θ°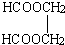 Θ§Ω…œ»÷Τ±ΗHCOOHΚΆHOCH2CH2OHΘ§Ω…“‘CH2CH=CH2ΈΣ‘≠ΝœΘ§―θΜ· «HCHOΚΆCH3CHOΘ§HCHO±Μ―θΜ·ΈΣHCOOHΘ§CH3CHO±ΜΜΙ‘≠ΈΣCH3CH2OHΘ§»ΜΚσCH3CH2OHΖΔ…ζœϊ»ΞΖ¥”Π…ζ≥…CH2=CH2Θ§CH2=CH2ΖΔ…ζΦ”≥…Ζ¥”Π…ζ≥…CH3BrCH2BrΘ§Υ°ΫβΩ……ζ≥…HOCH2CH2OHΘ§Εΰ’ΏΖΔ…ζθΞΜ·Ζ¥”ΠΩ……ζ≥…
Θ§Ω…œ»÷Τ±ΗHCOOHΚΆHOCH2CH2OHΘ§Ω…“‘CH2CH=CH2ΈΣ‘≠ΝœΘ§―θΜ· «HCHOΚΆCH3CHOΘ§HCHO±Μ―θΜ·ΈΣHCOOHΘ§CH3CHO±ΜΜΙ‘≠ΈΣCH3CH2OHΘ§»ΜΚσCH3CH2OHΖΔ…ζœϊ»ΞΖ¥”Π…ζ≥…CH2=CH2Θ§CH2=CH2ΖΔ…ζΦ”≥…Ζ¥”Π…ζ≥…CH3BrCH2BrΘ§Υ°ΫβΩ……ζ≥…HOCH2CH2OHΘ§Εΰ’ΏΖΔ…ζθΞΜ·Ζ¥”ΠΩ……ζ≥…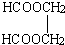 Θ§
Θ§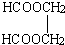 Θ§Ζ¥”ΠΒΡΖΫ≥Χ ΫΈΣ
Θ§Ζ¥”ΠΒΡΖΫ≥Χ ΫΈΣ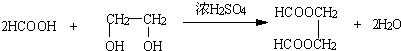 Θ§
Θ§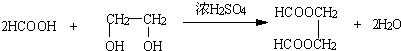 Θ°
Θ°

 » Α°”Δ”οΆ§≤ΫΝΖœΑ≤αœΒΝ–¥πΑΗ
» Α°”Δ”οΆ§≤ΫΝΖœΑ≤αœΒΝ–¥πΑΗ ―ßœΑ ΒΦυ‘ΑΒΊœΒΝ–¥πΑΗ
―ßœΑ ΒΦυ‘ΑΒΊœΒΝ–¥πΑΗ
