
 C��һ�������¿ɷ�����Ӧ������һ�ָ߷��ӻ�����E��E�Ľṹ��ʽΪ
C��һ�������¿ɷ�����Ӧ������һ�ָ߷��ӻ�����E��E�Ľṹ��ʽΪ ������R��R��Ϊ��������ش��������⣺
������R��R��Ϊ��������ش��������⣺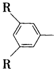 ����������������ͬ���칹��Ľṹ��ʽ
����������������ͬ���칹��Ľṹ��ʽ| 131 |
| 12 |
| 16 |
| 50% |
| 2��10+2-11-1 |
| 2 |
 ����BΪ
����BΪ ��AΪ
��AΪ ����DΪ
����DΪ ������л���Ľṹ�������Լ������Ӧ��Ϣ�����⣮
������л���Ľṹ�������Լ������Ӧ��Ϣ�����⣮| 131 |
| 12 |
| 16 |
| 50% |
| 2��10+2-11-1 |
| 2 |
 ����BΪ
����BΪ ��AΪ
��AΪ ����DΪ
����DΪ ��
��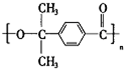 ��
�� ��
��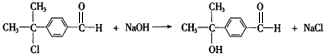 ��
�� ��
�� ����Ũ���ᡢ���ȵ������·�Ӧ��ȥ��Ӧ����F��C11H12O2����FΪ
����Ũ���ᡢ���ȵ������·�Ӧ��ȥ��Ӧ����F��C11H12O2����FΪ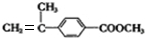 ��F�����Ӿ۷�Ӧ�ķ���ʽΪ��
��F�����Ӿ۷�Ӧ�ķ���ʽΪ��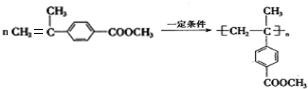 ��
��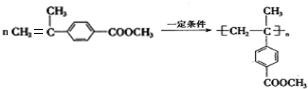 ��
�� �����ӽṹ�к���
�����ӽṹ�к��� ����������ͬ���칹���У�
����������ͬ���칹���У� ��
�� ��
��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����0.2mol/L��ijһԪ��HA��Һ��0.1mol/L NaOH��Һ�������Ϻ��ҺpH����7����Ӧ��Ļ��Һ��2c��OH-��+c��A-��=2c��H+��+c��HA�� |
| B��pH��Ϊ9��������Һ��CH3COOH��Na2CO3��NaOH�������ʵ���Ũ�ȵĴ�С˳����NaOH ��Һ��CH3COOH��Һ��Na2CO3��Һ |
| C��pH=3�Ķ�Ԫ����H2R��Һ��pH=11��NaOH��Һ��Ϻ��Һ��pH����7����Ӧ��Ļ��Һ��c��R2-��+c��HR-��=c��Na+�� |
| D��0.2mol/L NaHCO3��Һ��0.1mol/L NaOH��Һ�������ϣ�c��H+��=c��OH-��+c��HCO3-��+2c��H2CO3�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���٢ۢݢ� | B���ڢ� |
| C���ڢۢܢ� | D���٢ڢۢܢݢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ���� |


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
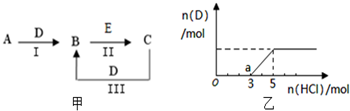 A��B��C��D��EΪ��ѧ��ѧ�����ĵ��ʻ���ת����ϵ��ͼ����ʾ
A��B��C��D��EΪ��ѧ��ѧ�����ĵ��ʻ���ת����ϵ��ͼ����ʾ�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| A��K��M��N���Ͽ�ʱ����װ������Ӧ���� |
| B��K��M��N���Ͽ�ʱ��Zn������Cu���� |
| C��K��M����ʱ��Cu2+��Zn���ƶ� |
| D��K��N����ʱ��Zn�ܽ⣬CuƬ�������� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com