

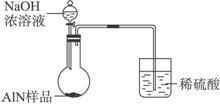
ͼ��
(1)ʵ���йز���Ϊ��
a.��Բ����ƿ�з���AlN��Ʒw g��������ƿ�м���ˮ��XҺ��
b.�ӷ�Һ©����Բ����ƿ�м���һ������Ĺ�����ŨNaOH��Һ
c.����װ�õ�������
d.��ȡ�ռ���ˮ�����
��ȷ�IJ���˳��Ϊ______________________��
(2)��ʵ���м��װ�������Եķ�����________________________��
(3)���ƿ�е��Լ�X��ѡ��___________(��ѡ��ı��)��
A.���� B.�ƾ� C.ֲ���� D.CCl4
(4)ʵ����������۲쵽��ƿ�л��й��壬����Ʒ�к��е�������________________��
(5)ʵ�������Һ©���е�NaOH��Һ�Ѿ�ȫ��������ƿ����Ͳ���ռ���ˮ�����Ϊa L������ʱ��ʵ������Ϊ��״��������Ʒ�е�AlN����������Ϊ___________(AlN��ʽ��Ϊ41)����һ�ⶨ�����ʵ��ֵƫ�ߣ�����Ϊ���ܵ�ԭ����__________________________��
(6)���˽������ͼ��װ�ý���ͬ��ʵ�飬��ͨ���ⶨ�ձ��������������ȷ����Ʒ��AlN����������������Ϊ�����ĸĽ��Ƿ���У�________________(����С������С�)��������________________________��
ͼ��
(1)c��a��b��d
(2)�رշ�Һ©������������Ͳ�м�����ˮ��û���ܿڣ���Բ����ƿ����Ͳ�в������ݣ��ָ�������ʱ�������γ�һ��Һ��
(3)C (4)̼
(5)![]() ��100% ����Ĺ�������������Һռ��һ���������ʹ�ų���ˮ�����ƫ��
��100% ����Ĺ�������������Һռ��һ���������ʹ�ų���ˮ�����ƫ��
(6)������ �������ױ��������գ�������������
����:�������⣬��ʵ��Ϊһ����ʵ�飬��˰�װ��ͼ����װ�ú�����Ҫ����װ�õ������ԣ��䷽���ǣ��رշ�Һ©������������Ͳ�м�����ˮ��û���ܿڣ�ʹװ���ܱգ�����Բ����ƿ��ʹװ��������������ͣ�����Ͳ�в������ݣ��ָ�������ʱ�������γ�һ��ˮ���������������á�����NH3��������ˮ����ˣ���NH3��ˮʱ���������Լ�X��NH3��H2O���������ƿ�е��Լ�X����߱������������ٲ���NH3��H2O��Ӧ�����ܽ�NH3��������ˮ�����ܶȱ�H2OС�������Լ�X��ѡ��ֲ���͡�ʵ�����������ƿ�л��й��壬����Ʒ�к��е�������̼����ΪAlN��Al2O3����NaOH��Һ��Ӧ���ܽ���NaOH��Һ�С�
��AlN+NaOH+H2O![]() NaAlO2+NH3��
NaAlO2+NH3��
![]() a L
a L
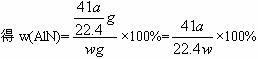
�ⶨ�����ʵ��ֵƫ�ߣ�����Ϊ����Ĺ���NaOH��Һռ��һ���������ʹ�ų���ˮ�����ƫ��aֵƫ��


 �����������ҵ�������������ϵ�д�
�����������ҵ�������������ϵ�д� ѧ���������ν��Ͼ���ѧ������ϵ�д�
ѧ���������ν��Ͼ���ѧ������ϵ�д� Happy holiday���ּ��������ҵ�㶫���������ϵ�д�
Happy holiday���ּ��������ҵ�㶫���������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������(AlN)��һ�����������ϣ��㷺Ӧ���ڼ��ɵ�·��������ij�������к���̼�����������ʣ�����ͼ��װ��������ʵ�飬ʹ��������Ʒ��NaOH��Һ��Ӧ��AlN+NaOH+H2O====NaAlO2+NH3�������ݷ�Ӧ�������ɰ�����������ⶨ��Ʒ�еĵ�����������������������ʵ��������ȷ�����ʵijɷ�(ʵ���е���������Բ���)��
(1)ʵ���йز���Ϊ��
A.����ƿ�з���������AlN��Ʒ b.�ӷ�Һ©������ƿ�м��������ŨNaOH
c.����װ�õ������� d.�ⶨ�ռ���ˮ�����
��ȷ�IJ���˳��Ϊ___________________________��
(2)��ʵ���м��װ�������Եķ�����__________________��
(3)���ƿ�е��Լ�XӦѡ��_________(��ѡ��ı��)��
A.���� B.�ƾ� C.ֲ���� D.CCl4
(4)���ƿ�ڵ�Һ��û��װ��(�Ϸ����������ռ�)��ʵ����NH3�������_________(�ƫ��ƫС�����䡱)��
(5)ʵ����������۲쵽��ƿ�л��й��壬����Ʒ�к��е�������_________��
(6)��ʵ���в����Ʒ������Ϊwg�����������Ϊa L(�����)������Ʒ��AlN����������Ϊ_________(AlN��ʽ��Ϊ41)��
(7)���˸���ͼ��װ�ý���ͬ��ʵ�飬ͨ���ⶨ�ձ��������������ȷ����Ʒ��A1N����������������Ϊ�Ƿ����?_________(����С������С�)��ԭ����_________���Ľ��ķ���Ϊ_________(����Ϊ�����С����˿ո���ش�)��
�鿴�𰸺ͽ���>>
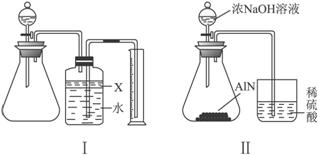
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(1)ʵ���йز���Ϊ��a.����ƿ�з���������AlN��Ʒ��b.�ӷ�Һ©������ƿ�м��������ŨNaOH��c.���װ�õ������ԣ�d.�ⶨ�ռ���ˮ���������ȷ�IJ���˳��Ϊ_____________��
(2)�������м��װ�������Եķ�����______________��
(3)���ƿ�е��Լ�X��ѡ��___________(��ѡ�����)��
A.���� B.�ƾ� C.ֲ���� D.���Ȼ�̼
(4)���ƿ��Һ��û��װ��(�Ϸ����������ռ�)��ʵ����NH3�������____________(�ƫ��ƫС�����䡱)��
(5)ʵ����������۲쵽��ƿ�л��й��壬����Ʒ�к��е�������__________________��
(6)��ʵ���в����Ʒ������Ϊw g�����������Ϊa L(��״��)������Ʒ��AlN����������Ϊ_______________(AlN����Է�������Ϊ41)��
(7)���˸���ͼ��װ�ý���ͬ����ʵ�飬ͨ���ⶨ�ձ��������������ȷ����Ʒ��AlN����������������Ϊ�Ƿ����____________(����С������С�)��ԭ����_________________���Ľ��ķ�����___________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ�긣��ʡ�����и���3��������⻯ѧ�Ծ��������棩 ���ͣ�ʵ����
������(AlN)��һ���������ǽ������ϡ�ijAlN��Ʒ������ֺAl2O3���ʣ�Ϊ�ⶨAlN�ĺ����������������ʵ�鷽��������֪��AlN+NaOH+H2O��NaAlO2��NH3����
������1��ȡһ��������Ʒ��������װ�òⶨ��Ʒ��AlN�Ĵ���(�г�װ������ȥ)��

��1����ͼCװ�������θ���ܵ�������______________��
��2���������ʵ�鲽�裺��װ��ʵ��װ�ã�����____________,�ټ���ʵ��ҩƷ����������ʵ�������______________,��Һ©������������NaOHŨ��Һ�������ٲ������塣��K1��ͨ�뵪��һ��ʱ�䣬�ⶨCװ�÷�Ӧǰ��������仯��ͨ�뵪����Ŀ����______________��
��3������װ�ô���ȱ�ݣ����²ⶨ���ƫ�ߣ�������Ľ����___________��
������2������ͼװ�òⶨm g��Ʒ��A1N�Ĵ���(���ּг�װ������ȥ)��
(
��4��Ϊ�ⶨ������������������װ���е�XҺ�������_________________________��
a��CCl4????????? b��H2O???? c��NH4Cl��Һ??? d��
��5����m g��Ʒ��ȫ��Ӧ�����������������ΪV mL(��ת��Ϊ��״��)����AIN����������__��
������3�������²���ⶨ��Ʒ��A1N�Ĵ��ȣ�

��6����������ɳ��������ӷ���ʽΪ___________________��
��7�����ڲ������δϴ�ӣ��ⶨ�����__________(�ƫ��������ƫ�͡�����Ӱ����)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
Al2O3+![]() 2AlN+3CO
2AlN+3CO
������(AlN)��һ�����������ϣ��㷺Ӧ���ڼ��ɵ�·��������
(1)���ݵ���������ȡԭ�����Ʋ�ij���������������ʿ�����__________(�м��־�д����)��
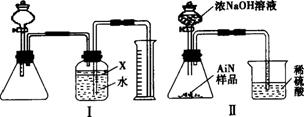
(2)ijͬѧ����ͼ���е�һЩװ�����ⶨ������������������ʹ��������Ʒ��NaOH��Һ��Ӧ����NaAlO2�����ų����������ݷ�Ӧ�������ɰ�����������ⶨ��Ʒ�е�����������������������ʵ��������ȷ�����ʵijɷ�(ʵ���е���������Բ���)

ͼ5-2
��д����������NaOH��Һ��Ӧ�Ļ�ѧ����ʽ��______________________________��
�ڱ������м��ͼI��װ�������Եķ�����_________________________________��
�۹��ƿ�е��Լ�X���ѡ��___________(��ѡ��ı��)
A.�� B.�ƾ� C.ֲ���� D.CCl4
�ܹ��ƿ��Һ��û��װ��(�Ϸ����������ռ�)��ʵ����NH3�������___________(�ƫ����ƫС�����䡱)
����ʵ���в����Ʒ������Ϊ10.
��ʵ����������۲쵽��ƿ�л��й��壬����Ʒ�е����ʺ�___________��Ϊ�˲ⶨ�Ƿ����������ʣ�����Ҫ��Щ�����ݣ�___________��
�߸�ͬѧʵ�鷽��������������������������������ϴ����˽������ͼ5-2�����ҡ�������װ���е�һ��(��ͨ����ڽ���)��ֻ����м��ֱ�Ҫ�����ݲⶨ���ɱȽ�ȷ��ȷ����Ʒ��AlN�������������Ϻ�����װ���ǣ�___________(�����)��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com