
| ����/��mol��L-1�� | Pb2+ | Ca2+ | Fe3+ | Mn2+ | Cl- |
| ����ǰŨ�� | 0.100 | 29.8 | 0.120 | 0.087 | 51.9 |
| ������Ũ�� | 0.004 | 22.6 | 0.040 | 0.053 | 49.9 |
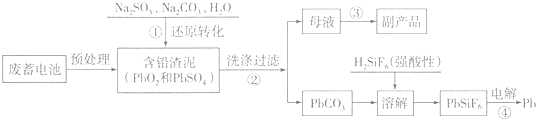
���� ��1��PbSO4ת��ΪPbCO3����ʽΪ��PbSO4��s��+CO32-��aq��?PbCO3��s��+SO42-��aq��������ʹ�Һ���Ũ���Ƕ�ֵ�������ڱ���ʽ�У�
��ֽ��裬�ʵ������¶ȣ�����̼���Ƶ�Ũ�ȵȿ��Լӿ췴Ӧ���ʣ�
��2�������PbO2���������Ʒ���������Ӧ����PbSO4�����ݵ���غ㣬��֪������NaOH��
��3������������ԭ��Ӧ��Pb2+��õ�������Pb��
��4�����ȷֽ���̹�����PbԪ���������䣻
��5������ȥ����=$\frac{����Ũ�ȱ仯��}{������ʼŨ��}$��100%��ȥ����Խ��ȥ��Ч��Խ�ã�
�μӷ�Ӧ����Pb2+����ͼ���֪��ѡ��PHҪʹǦ��Pb2+��ʽ���ڣ�����̫ǿ������Ӧ�ᱻ���ƣ�
��� �⣺��1��PbSO4ת��ΪPbCO3����ʽΪ��PbSO4��s��+CO32-��aq��?PbCO3��s��+SO42-��aq����ƽ�ⳣ��K=$\frac{c��S{{O}_{4}}^{2-}��}{c��C{{O}_{3}}^{2-}��}$��Ϊ�˼ӿ췴Ӧ���ʺ�Ǧ�����ʣ����Բ�ȡ��ʩ�У���ֽ��裻�ʵ������¶ȣ�����̼���ƺ��������Ƶ�Ũ�ȵȣ�
�ʴ�Ϊ��$\frac{c��S{{O}_{4}}^{2-}��}{c��C{{O}_{3}}^{2-}��}$����ֽ��裻�ʵ������¶ȣ�����̼���ƺ��������Ƶ�Ũ�ȣ���д���㣩��
��2�������PbO2���������Ʒ���������Ӧ����PbSO4�����ݵ���غ㣬��֪������NaOH����Ӧ���ӷ���Ϊ��PbO2+SO32-+H2O=PbSO4+2OH-��
�ʴ�Ϊ��PbO2+SO32-+H2O=PbSO4+2OH-��
��3������������ԭ��Ӧ��Pb2+��õ�������Pb�������缫��Ӧʽ��Pb2++2e-=Pb��
�ʴ�Ϊ��Pb2++2e-=Pb��
��4�����ȷֽ���̹�����PbԪ���������䣬��100��$\frac{207}{207+32}$=96��$\frac{207}{207+16x}$�����x=1.4��
�ʴ�Ϊ��1.4��
��5��Fe3+ȥ����Ϊ$\frac{0.12-0.04}{0.12}$��100%=67%��Ca2+ȥ����Ϊ$\frac{29.8-22.6}{29.8}$��100%=24%��Mn2+ȥ����Ϊ$\frac{0.087-0.053}{0.087}$��100%=39%��Cl-ȥ����Ϊ$\frac{51.9-49.9}{51.9}$��100%=3.9%�����Pb2+�⣬����Ǧ�����������ӵ�ȥ��Ч����õ���Fe3+��
��ӦΪ2EH��s��+Pb2+?E2Pb��s��+2H+���μӷ�Ӧ����Pb2+����ͼ���֪��ѡ��PHҪʹǦ��Pb2+��ʽ���ڣ�����̫ǿ������Ӧ�����ƣ�ȥ��Ч�����ͣ�����Ǧʱ����ʵ�pHԼ6��7��
�ʴ�Ϊ��B��
���� �����Դӷ�Ǧ���ص�Ǧ�����ǦΪ���壬������Ϣ��ȡ��Ǩ�����á�İ������ʽ����д����Ӧ����Ӱ�����ء����ԭ������������ͼ��ķ��������ȣ��Ѷ��еȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2017�����ʡ�����и�����ѧ��10�¶�λ���Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
����������Ʊ�������ʵ���У�������ó��Ľ��۴������
ѡ�� | �Լ� | ��ֽ����Һ | ���� | ���� |
A | �������ơ����� | Ʒ����Һ | ��ɫ | SO2���л�ԭ�� |
B | Ũ���ᡢŨ���� | pH��ֽ | ��� | HClΪ�������� |
C | Ũ���ᡢ�������� | ���۵⻯����Һ | ���� | Cl2���������� |
D | Ũ��ˮ����ʯ�� | ��ɫʯ����ֽ | ���� | NH3Ϊ�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ij��ѧʵ��С����Ҫ�˽��г�������ʳ�ð״ף���Ҫ�Ǵ����ˮ��Һ������ȣ��ִ��г�������һƿijƷ��ʳ�ð״ף���ʵ���ұ�NaOH��Һ������еζ����±���4�ֳ���ָʾ���ı�ɫ��Χ��
ij��ѧʵ��С����Ҫ�˽��г�������ʳ�ð״ף���Ҫ�Ǵ����ˮ��Һ������ȣ��ִ��г�������һƿijƷ��ʳ�ð״ף���ʵ���ұ�NaOH��Һ������еζ����±���4�ֳ���ָʾ���ı�ɫ��Χ��| ָʾ�� | ʯ�� | ���� | ���� | ��̪ |
| ��ɫ��Χ��pH�� | 5.0��8.0 | 3.1��4.4 | 4.4��6.2 | 8.2��10.0 |
| ʵ����� | ��һ�� | �ڶ��� | ������ |
| ����NaOH��Һ���/mL | 26.02 | 25.32 | 25.28 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| �������� | ��ʼ������pH | ��ȫ������pH |
| Fe3+ | 1.1 | 2.8 |
| A13+ | 3.4 | 4.7 |
| Fe2+ | 5.8 | 8.8 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| ���� | Cu2- | Al3+ | H+ | Cl- | SO42- |
| c��mol/L�� | 1 | 1 | 2 | 3 | a |
| A�� | ��·�й�ת��0.9mol���� | B�� | ��Ԫ����Al��OH��3����ʽ���� | ||
| C�� | ������������3.2g | D�� | a=2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | W��X��Y��Zԭ�ӵĺ����������������ܺ�Ϊ19 | |
| B�� | W�������Ӱ뾶С��Li+ | |
| C�� | W��Y���γɼȺ����Ӽ��ֺ����ۼ��Ļ����� | |
| D�� | X��Y�ļ���̬�⻯����ȶ��ԣ�X��Y |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ѡ�� | ���� | Ҫ�� |
| A | K+��Cl-��SO42-��MnO4- | c��K+����c��Cl-�� |
| B | Na+��Ca2+��I-��NO3- | c��H+��/c��OH-��=1��1014 |
| C | Al3+��NH4+��SO42-��CH3COO- | �μ�NaOH��Һ������������� |
| D | Na+��Mg2+��Ag+��NO3- | �μӰ�ˮ���г�������������������ܽ� |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ù���ʯ�������չ�ҵβ���е�SO2��Ca2++2OH-+SO2�TCaSO3��+H2O | |
| B�� | ������KMnO4��Һ��H2O2��Ӧ��֤��H2O2���л�ԭ�ԣ�2MnO4-+6H++5H2O2�T2Mn2++5O2��+8H2O | |
| C�� | ��ͭ���缫���NaCl��Һ��2C1-+2H2O$\frac{\underline{\;����\;}}{\;}$H2��+Cl2��+2OH- | |
| D�� | ��Fe2O3���뵽HI��Һ�У�Fe2O3+6H+�T2Fe3++3H2O�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com