
 ��Ԫ��e�ĵ����Ų�ʽΪ1s22s22p63s23p5��c�����ӽṹʾ��ͼΪ
��Ԫ��e�ĵ����Ų�ʽΪ1s22s22p63s23p5��c�����ӽṹʾ��ͼΪ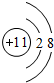
 ��
������ ԭ��������������Ķ�����Ԫ��a��b��c��d��e�У�a������������Ϊ���������Ķ�������a��2�����Ӳ㣬����������Ϊ4����aΪ̼Ԫ�أ�b��d��A2B���⻯���ΪV�η��ӣ�bΪ��Ԫ�أ�dΪ��Ԫ�أ�c��+1�����ӱ�e��-1��������8�����ӣ�cΪ��Ԫ�أ�eΪClԪ�أ�
��� �⣺ԭ��������������Ķ�����Ԫ��a��b��c��d��e�У�a������������Ϊ���������Ķ�������a��2�����Ӳ㣬����������Ϊ4����aΪ̼Ԫ�أ�b��d��A2B���⻯���ΪV�η��ӣ�bΪ��Ԫ�أ�dΪ��Ԫ�أ�c��+1�����ӱ�e��-1��������8�����ӣ�cΪ��Ԫ�أ�eΪClԪ�أ�
��1��Ԫ��aΪ̼Ԫ�أ������Ų�ͼΪ ��Ԫ��eΪCl�������Ų�ʽΪ 1s22s22p63s23p5��c������ΪNa+�����ӽṹʾ��ͼΪ
��Ԫ��eΪCl�������Ų�ʽΪ 1s22s22p63s23p5��c������ΪNa+�����ӽṹʾ��ͼΪ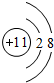 ��
��
�ʴ�Ϊ�� ��1s22s22p63s23p5��
��1s22s22p63s23p5�� ��
��
��2������ЩԪ�����γɵ�˫ԭ�ӻ���������У�һ����̼��
�ʴ�Ϊ��һ����̼��
��3��������Ԫ����ɵ���ԭ�ӷ����У����ӵĿռ�ṹ����ֱ���ε���CO2��CS2��
�ʴ�Ϊ��CO2��CS2��
��4����ЩԪ���γɵ�AB�ͻ������У��侧�������������Ӿ������NaCl�����ڷ��Ӿ������CO���õ���ʽ��ʾNaCl���Ӿ�����γɹ��� ��
��
�ʴ�Ϊ��NaCl��CO�� ��
��
��5��Ԫ��c��e�γɵĻ�����ΪNaCl��NaCl��ˮ��Һ�����ڹ�ҵ�����������÷�Ӧ�Ļ�ѧ����ʽΪ��2NaCl+2H2O$\frac{\underline{\;���\;}}{\;}$2NaOH+H2��+Cl2����
�ʴ�Ϊ��2NaCl+2H2O$\frac{\underline{\;���\;}}{\;}$2NaOH+H2��+Cl2����
���� ���⿼��ṹ����λ�ù�ϵӦ�ã��ƶ�Ԫ���ǽ���ؼ������ػ�ѧ���������ṹ�Ŀ��飬�Ѷ��еȣ���Ҫѧ���������ջ���֪ʶ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 �����õ���ʽ��ʾԭ�Ӹ�����Ϊ2��1�ĸû�������γɹ���
�����õ���ʽ��ʾԭ�Ӹ�����Ϊ2��1�ĸû�������γɹ��� ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ���� | ������������ | ������g�� | ���ʵ�����mol�� | Ħ��������g/mol�� |
| ���� | 0.3 | |||
| S | 1.204��1023 | |||
| CO2 | 22 | |||
| C12H22O11 | 0.25 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

| ���볣�� �� | K1 | K2 |
| H2SO3 | 1.3��10-2 | 6.3��10-8 |
| H2CO3 | 4.2��10-7 | 5.6��10-11 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ij��ȤС��ͬѧ�о�������Ԫ�ؼ���ijЩ������IJ������ʣ������������£�
ij��ȤС��ͬѧ�о�������Ԫ�ؼ���ijЩ������IJ������ʣ������������£��鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com