

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014��㶫ʡ��ʮУ��������һ��������ѧ�Ծ��������棩 ���ͣ������
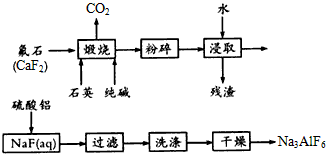
����һ��Ӧ�ù㷺�Ľ�������ҵ����Al2O3�ͱ���ʯ��Na3AlF6��������ڵ���Ƶá����������Ҫ�ɷ���Al2O3��SiO2������������NaOH��Һ�����ʡ���������������Al2O3���������£�
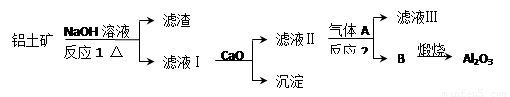
�ش��������⣺
��1��д����Ӧ1�Ļ�ѧ����ʽ ��
��2����Һ���м���CaO���ɵij����� ����Ӧ2�����ӷ���ʽΪ ��
��3����������Ļ�ѧ����ʽ�� ����ʯīΪ�缫�����������Ļ������ijɷ��� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����һ��Ӧ�ù㷺�Ľ�������ҵ����Al2O3�ͱ���ʯ��Na3AlF6��������ڵ���Ƶá����������Ҫ�ɷ���Al2O3��SiO2������������NaOH��Һ�����ʡ���������������Al2O3���������£�

�ش��������⣺
��1��д����Ӧ1�Ļ�ѧ����ʽ ��
��2����Һ���м���CaO���ɵij����� ����Ӧ2�����ӷ���ʽΪ ��
��3����������Ļ�ѧ����ʽ�� ����ʯīΪ�缫�����������Ļ������ijɷ��� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����һ��Ӧ�ù㷺�Ľ�������ҵ����Al2O3�ͱ���ʯ��Na3AlF6��������ڵ��
�Ƶá����������Ҫ�ɷ���Al2O3��SiO2������������NaOH��Һ�����ʡ���������������Al2O3���������£�
 |
�ش��������⣺
��1��д����Ӧ1�Ļ�ѧ����ʽ �� ��
��2����Һ���м���CaO���ɵij����� ����Ӧ2�����ӷ���ʽΪ ��
��3����������Ļ�ѧ����ʽ�� ����֪AlCl3���۵��Al2O3���۵�͵ö࣬��ҵ�����е��Al2O3��������ұ���������ǵ�����ڵ�AlCl3��ԭ���ǣ� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com