

 $\stackrel{����}{��}$
$\stackrel{����}{��}$ +R3COOH
+R3COOH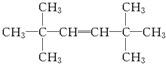 G��
G�� ��
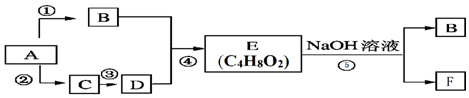
�� ���� ��1��A�IJ���ͨ������һ�����ҵ�ʯ�ͻ���ˮƽ����AӦΪCH2=CH2��B��D����E����E�ķ���ʽ��֪EΪCH3COOC2H5��CH3COOC2H5��NaOH��Һ����ˮ�ⷴӦ����CH3CH2OH��CH3COONa������BΪCH3CH2OH��FΪCH3COONa�����ת����ϵͼ��֪��A����������������Ӧ����CH3CHO��CH3CHO�ɽ�һ����������CH3COOH����CΪCH3CHO��DΪCH3COOH��
��2����A��N��C��=$\frac{140��0.857}{12}$=10��N��H��=$\frac{140-12��10}{1}$=20����A�ķ���ʽΪC10H20��A������������̼ԭ�Ӳ�����ԭ��ֱ��������˵��������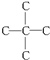 �Ľṹ��A��һ������������ֻ����G��G��ʹʯ����Һ��죬��G�����Ȼ���˵��A��������
�Ľṹ��A��һ������������ֻ����G��G��ʹʯ����Һ��죬��G�����Ȼ���˵��A�������� ����Ϊ�Գƽṹ����A�Ľṹ��ʽΪ
����Ϊ�Գƽṹ����A�Ľṹ��ʽΪ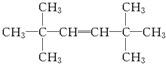 ���ݴ˽��
���ݴ˽��
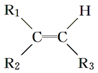
��� �⣺��1��a���������Ϸ�����AΪΪCH2=CH2��������̼ԭ����̼ԭ��ͨ�����Թ��õ��Ӷ������ӣ�̼ԭ������ԭ��ͨ��һ�Թ��õ��Ӷ������ӣ�����CH2=CH2�ĵ���ʽΪ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
b����������Ʒ�Ӧ�ų������������ǻ������Ȼ������Է���������������B��D���ʴ�Ϊ��B��D��
c����Ӧ��Ϊ�Ҵ������ᷢ��������Ӧ��Ӧ������������Ӧ�Ļ�ѧ����ʽΪCH3COOH+C2H5OH$?_{��}^{Ũ����}$CH3COOC2H5+H2O��
�ʴ�Ϊ��CH3COOH+C2H5OH$?_{��}^{Ũ����}$CH3COOC2H5+H2O��
��2����A��N��C��=$\frac{140��0.857}{12}$=10��N��H��=$\frac{140-12��10}{1}$=20����A�ķ���ʽΪC10H20��A������������̼ԭ�Ӳ�����ԭ��ֱ��������˵�������� �Ľṹ��A��һ������������ֻ����G��G��ʹʯ����Һ��죬��G�����Ȼ���˵��A��������
�Ľṹ��A��һ������������ֻ����G��G��ʹʯ����Һ��죬��G�����Ȼ���˵��A�������� ����Ϊ�Գƽṹ����A�Ľṹ��ʽΪ
����Ϊ�Գƽṹ����A�Ľṹ��ʽΪ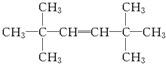 ��������Ϣ��Ӧ����GΪ
��������Ϣ��Ӧ����GΪ ��
��
�ʴ�Ϊ��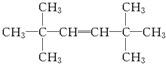 ��
�� ��
��
���� ������Ҫ�����л����ƶ��Լ��л������ʽ��ṹʽ��ȷ���ȣ��ѶȲ���ע�����֪ʶ���������գ���2��ע������л���Ľṹ�ص��������ж�A�Ľṹ��


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 2Na2O2��s��+2CO2�� s��=2Na2CO3��s��+O2��g����H��-452 kJ/mol | |
| B�� | CO��ȼ����Ϊ283 kJ | |
| C�� | 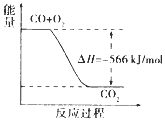 ��ͼ�ɱ�ʾ��CO����CO2�ķ�Ӧ���̺�������ϵ | |
| D�� | CO��g����Na2O2��s����Ӧ�ų�509kJ����ʱ������ת����Ϊ6.02��l023 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ������Ϊ���� | ʵ������� | |
| A | NO2��NO�� | ͨ��O2 |
| B | CuSO4��Һ���������� | ��������CuO������ |
| C | �屽���壩 | ����NaOH��Һ����Һ |
| D | MnO2��I2�� | ���� |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �����£�10g46%��CH3CH2OH��Һ�������е���ԭ����Ϊ0.4NA | |
| B�� | ��FeI2��Һ��ͨ��������Cl2������1molFe2+������ʱ����ת�Ƶ�����ĿΪ3NA | |
| C�� | �ڱ�״���£���11.2LHCl����1Lˮ�У���Һ��HCl������Ϊ0.5NA | |
| D�� | ���³�ѹ�£�0.1molNaHSO4�����У�����������������0.3NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ˮ | B�� | ���� | C�� | ���� | D�� | ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������ˮ��ˮϡ�ͣ���ϡ�����У�$\frac{c��N{H}_{4}^{+}��}{c��N{H}_{3}•{H}_{2}O��}$ʼ�ձ������� | |
| B�� | �μӹ����У�����Һ��c��Cu2+��=2.2��10-2mol/L ʱ����Һ��pH=9 | |
| C�� | �μӹ����У�����Һ��pH=7 ʱ����Һ��2c��NH4+��=c��SO42-�� | |
| D�� | ���μ�pH=11 ��NaOH ��Һ��Cu2+��ȫ����ʱ������Һ�����С��V |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����Ԫ����ɵľ���һ��ֻ�����Լ� | |
| B�� | ���Ӿ���һ�������Ǽ��Լ� | |
| C�� | ԭ�Ӿ���һ�����й��ۼ� | |
| D�� | ���Ӿ���һ�����й��ۼ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | ��״���£�2.24L����Լ����3.612��1023��̼ԭ�� | |
| B�� | ���³�ѹ�£������ͳ����Ļ����16g��Լ����6.02��1023����ԭ�� | |
| C�� | 25��ʱ��1 L pH=13������������Һ��Լ����6.02��l023������������ | |
| D�� | 0.5mol CH4��Լ����3.01��1024������ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com