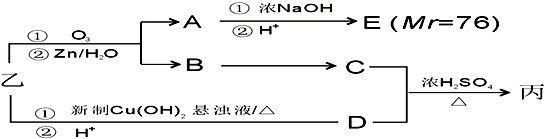
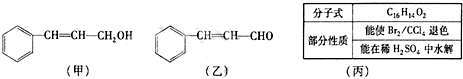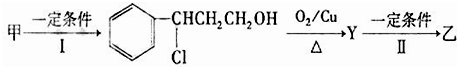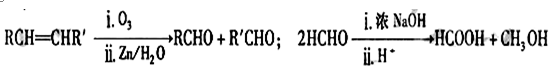���������μ����ֽ��Ҳ���ϸ��ӣ�ijѧϰС����Mg��NO
3��
2Ϊ�о�������ͨ��ʵ��̽�����ȷֽ�IJ���������4�ֲ��룺
�ף�Mg��NO
3��
2��NO
2��O
2 �ң�MgO��NO
2��O
2 ����Mg
3N
2��O
2 ����MgO��NO
2��N
2��1��ʵ��ǰ��С���Ա�������϶����붡��������������
��
�������ϵ�֪��2NO
2+2NaOH�TNaNO
3+NaNO
2+H
2O����Լס��ҡ������룬�����ͼ��ʾ��ʵ��װ�ã�ͼ�м��ȡ��г������Ⱦ�ʡ�ԣ���

��2��ʵ����̣�
��ȡ�����Ӻ��˹����Լ�֮ǰ���ر�k����Ӳ�ʲ����ܣ�A�����۲쵽E �������������ų�������
��
�ڳ�ȡMg��NO
3��
2����3.79g����A�У�����ǰͨ��N
2������װ���ڵĿ�������Ŀ���DZ���Բ���O
2�ļ���������ţ��ر�K���þƾ��Ƽ���ʱ����ȷ��������
Ȼ��̶��ڹ��й��岿λ�¼��ȣ�
�۹۲쵽A���к���ɫ������֣�C��D��δ�����Ա仯��
�ܴ���Ʒ��ȫ�ֽ⣬Aװ����ȴ�����¡����������ʣ����������Ϊ1.0g
��ȡ����ʣ��������Թ��У���������ˮ��δ����������
��3��ʵ������������
�ٸ���ʵ�������ʣ�����������������ɳ���ȷ�ϲ���
����ȷ�ģ�
�ڸ���D������������һλͬѧ��Ϊ����ȷ�Ϸֽ��������O
2��������O
2��D�н�����������ԭ��Ӧ��
����д��ѧ����ʽ������Һ��ɫ����ȥ��С�������϶��ֽ��������O
2���ڣ�δ��ൽ��ԭ����O
2��ͨ��װ��Bʱ�Ѳ��뷴Ӧ��
��С�����ۺ��ɵĹ�ʶ������ʵ������Բ����ƣ���Ľ�װ�ý�һ���о���

 Һ̬HCl
Һ̬HCl NaOH����
NaOH���� Һ̬HCl
Һ̬HCl NaOH���嶼�ǻ������ˮ��Һ��������״̬���ܹ��������ڵ���ʣ�
NaOH���嶼�ǻ������ˮ��Һ��������״̬���ܹ��������ڵ���ʣ�
 ���ڢޢ⣻
���ڢޢ⣻

 Ʒѧ˫�ž�ϵ�д�
Ʒѧ˫�ž�ϵ�д� Сѧ��ĩ���100��ϵ�д�
Сѧ��ĩ���100��ϵ�д� ��ĩ��ϰ���ϵ�д�
��ĩ��ϰ���ϵ�д�