
| ��� | �������� | ʵ��Ŀ�� |
| A | | ̽�����ڸ�������е�������� |
| B | ����������ע������ˮ��û����������ֲ����Һ�� | |
| C | | ̽�������п�����ˮ��ʱ��������� |


 �ŵ������ϵ�д�
�ŵ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
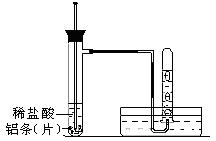
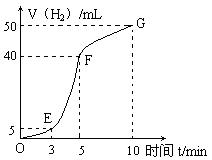
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| ʵ����� | Ԥ������ͽ��� |
| | |
| | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����������ˮ�к���Cl�� | B�����鼦�����к���̼���� |
| C������ӵ�ʳ���мӵIJ��ǵⵥ�� | D����ȥ��ˮƿ�е�ˮ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
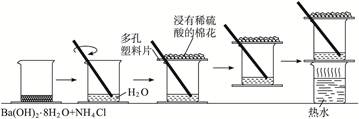
| ʵ�鲽�� | ʵ������ | �ó����� |
| �������Ϻ������ò��������ٽ������� | �д̼�����ζ�������������������ʹʪ�����ɫʯ����ֽ���� | |
| ���ִ����ձ��²� | �о��ձ����� | |
| ���������ձ� | �ձ�����Ĵ��м���ˮ�IJ���Ƭ(��Сľ��)ճ�����ձ��ײ� | |
| ��ճ�в���Ƭ���ձ�����ʢ����ˮ���ձ���һ��������� | ����Ƭ���������ձ��ײ� | |
| ��Ӧ��������ձ��ϵĶ������QƬ���۲췴Ӧ�� | �����ɺ�״ | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������ƿ��������ˮ |
| B��������KOH�������ձ��� |
| C������ʱ���� |
| D������ʱ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����������茶�����˺�����ˮ�Ҵ�ϴ�ӣ���˾ƥ�־�����˺���ˮϴ�� |
| B���ڡ����ͷ����Ԫ�صļ��顱ʵ���У�ժ�¼���δȼ���Ļ��ͷ���������ˮ�У��Ժ�ȡ������Һ���Թ��У��μ���������Һ��ϡ��������ж���Ԫ�صĴ��� |
| C���к͵ζ�ʵ���У�����ƿ����ƿ������ˮϴ����ʹ�ã��ζ��ܺ���Һ��������ˮϴ������������ϴ��ʹ�� |
| D��ֽ����ʵ������ѡ��ˮ���̶��࣬�л��ܼ��������࣬����ˮ��ǿ�ijɷ����������з������һЩ����ëϸ���������£����������ƶ����ٶ���һЩ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com