
| ��ѧʽ | CH3COOH | H2CO3 | HClO |
| ����ƽ�ⳣ�� | 1.8��10��5 | K1 4.3��10��7 K2 5.6��10��11 | 3.0��10��8 |
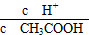 C��c(H��)��c(OH��) D.
C��c(H��)��c(OH��) D.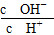


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��ˮ�����ӻ�����KWֻ���¶��йأ�������ᡢ���һ����Ӱ��ˮ�ĵ���̶� |
| B��Ksp���������ܵ���ʵ����ʺ��¶��йأ�������Һ��������ӵ�Ũ���й� |
| C�������£���0.10 mol��L��1��NH3��H2O��Һ�м�������NH4Cl���壬��ʹ��Һ��pH��С��c��NH4+��/c��NH3��H2O����ֵ���� |
| D�������£�CH3COOH��KW��1.7��10��5��NH3��H2O��Kb��1.7��10��5��CH3COOH��Һ�е�c��H������NH3��H2O�е�c��OH������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���ֱ��ˮϡ����ͬ�ı�����ϡ�ͺ�����ҺpH����ͬ |
| B��NaOH��Һ��ˮ���������c(OH��)��С |
| C���õ�Ũ�ȵ�������ȫ�кͣ��������������� |
| D����������NH4Cl(s)��pH����С |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
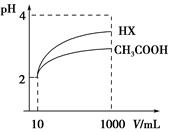
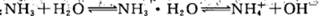 �ı�������������ʹ����̶��������
�ı�������������ʹ����̶��������| A����Ũ��ˮ | B�������¶� | C����NH4Cl��Һ | D����NaOH��Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����pH=5��H2SO4��Һϡ��1��103��,c(H+):c(SO42-)=2:1 |
B������������Ƶ�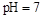 �Ļ����Һ��:c(CH3COO-)+c(CH3COOH)<c(Na+) �Ļ����Һ��:c(CH3COO-)+c(CH3COOH)<c(Na+) |
C�� ���������Һ��:c(NH4+)+c(H+)��c(SO42-)+c(OH-) ���������Һ��:c(NH4+)+c(H+)��c(SO42-)+c(OH-) |
| D��������pH=4��NaHC2O4��Һ��:c(H2C2O4)<c(C2O42��) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| ʵ���� | HA���ʵ���Ũ��(mol��L-1) | NaOH���ʵ���Ũ��(mol��L-1) | �����Һ ��pH |
| a | 0.1 | 0.1 | pH��9 |
| b | c | 0.2 | pH��7 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��m��n | B��m��n | C��m��n | D������ȷ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��

| A������IΪ����ϡ��ʱpHֵ�仯���� |
| B��a��ʱ������������ͬ��С��п������ʱ���ᷴӦ�����ʴ� |
| C��a��ʱ����������������п������������ᷴӦ������������ |
| D��b����Һ��ˮ�ĵ���̶ȱ�c����Һ��ˮ�ĵ���̶�С |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com