
| ���� |
| ���� |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����


| ||
| ||
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
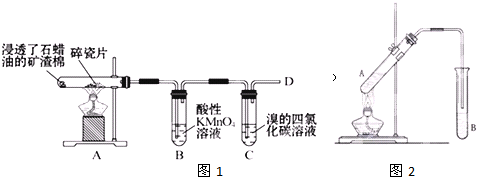
 ʯ���ͣ�17��̼ԭ�����ϵ�Һ̬���������ķֽ�ʵ��װ����ͼ��ʾ������������ʡ�ԣ������Թܢ��м���ʯ���ͺ�����������ʯ���ֽ⣩���Թܢڷ�����ˮ�У��Թܢ��м�����ˮ��
ʯ���ͣ�17��̼ԭ�����ϵ�Һ̬���������ķֽ�ʵ��װ����ͼ��ʾ������������ʡ�ԣ������Թܢ��м���ʯ���ͺ�����������ʯ���ֽ⣩���Թܢڷ�����ˮ�У��Թܢ��м�����ˮ��| ���� |
| �� |
| ���� |
| �� |

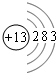
 ��CH3-CH2=CH2+Br2--��CH3-CH2-CH2
��CH3-CH2=CH2+Br2--��CH3-CH2-CH2 ��CH3-CH2=CH2+Br2--��CH3-CH2-CH2
��CH3-CH2=CH2+Br2--��CH3-CH2-CH2�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(1)��ͼ1-5-15����ѡ�����������������ȡ����NH3��װ�ü�ͼ(���ӱ�Ҫ�����ӡ��������ܡ���Ƥ�ܡ��̶�װ�ú�β������װ�ò��û�)�������������Լ���
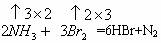
ͼ1-5-15
(2)��NH3ͨ����ˮ�У���N2���ɣ���Ӧ�Ļ�ѧ����ʽΪ__________________��
(3)Ϊ��֤����ͬ��ͬѹ�£���ͬ������κ����嶼������ͬ����Ŀ�ķ��ӡ�����С��ͬѧ���������ͼ1-5-16��ʾ��װ�ã�ͼ��B�ܵ��ݻ���A�ܵ�2��������K1��K2��K3��K4��K5���ر�(�̶�װ�ú�β������װ���ԣ�HCl��NH3��������ʯ���ͣ�Ҳ����֮��Ӧ��װ�������Ժ�)��
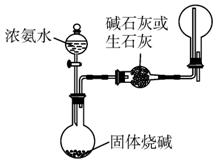
ͼ1-5-16
����A���г��������ѹǿ��ȵĸ���HCl���塣������__________________�����ƻ���K4��K5������C��ʹB�ܳ�����A��ͬѹ�ĸ���NH3��
�ڻ�����������K3��A���е�������__________________��Ҫ�ﵽʵ��Ŀ�ģ�����Ӧ��ɲ��ָ�������ʱ��B����Ԥ�ڵ�������__________�����۲첻��Ԥ��������Ҫԭ����_____________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���Ϻ��б�ɽ��������ѧ�߶����ϣ���ĩ��ѧ�Ծ��������棩 ���ͣ������
 C8H18+C9H18 C8H18
C8H18+C9H18 C8H18 C4H10+C4H8
C4H10+C4H8

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com