
 5NaC1+NaC1O3+3H2O�����ڡ�5���NaOH��Һ��ͨ������C12��ƽ�ⳣ��K=1.09��1012������ʱC12������������Ҫ�� ������Һ���ȣ���Һ����Ҫ����Ũ�����¶ȵı仯����ͼ��ʾ��ͼ��A��B��C���α�ʾ�������� ��
5NaC1+NaC1O3+3H2O�����ڡ�5���NaOH��Һ��ͨ������C12��ƽ�ⳣ��K=1.09��1012������ʱC12������������Ҫ�� ������Һ���ȣ���Һ����Ҫ����Ũ�����¶ȵı仯����ͼ��ʾ��ͼ��A��B��C���α�ʾ�������� ��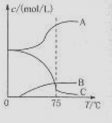
 NaC1O3+3H2��
NaC1O3+3H2�� HC1O+H++C1�� HC1O
HC1O+H++C1�� HC1O H++C1O��
H++C1O��

 5NaC1+NaC1O3+3H2O��NaCLO���ϼ�С����NaCL����������NaCLO3��������
5NaC1+NaC1O3+3H2O��NaCLO���ϼ�С����NaCL����������NaCLO3��������

 ��ĩ�óɼ�ϵ�д�
��ĩ�óɼ�ϵ�д� 99��1������ĩ��ѵ��ϵ�д�
99��1������ĩ��ѵ��ϵ�д� ��ǿ��У��ĩ���100��ϵ�д�
��ǿ��У��ĩ���100��ϵ�д� �óɼ�1��1��ĩ���100��ϵ�д�
�óɼ�1��1��ĩ���100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
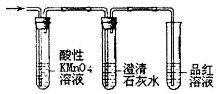
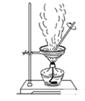
| A������ȩ���뺬������������Һ���Թ��У��ڷ�ˮԡ�м�����ֵ������ |
| B������12.5gCuSO4��5H2O�����100mL��Һ���õ�0��5mol/L��CuSO4��Һ |
| C����ͼ1װ�ü���ʵ�����Ƶõ���ϩ�л���SO2��CO2 |
| D������ͼ2װ�ô��Ȼ�����Һ��ֱ�������ᾧ����Ȼ������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
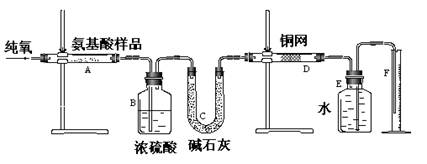
| A�����ɶ�����̼��������� | B������ˮ������ |
| C��ͨ����������� | D�����������Է������� |
�鿴�𰸺ͽ���>>
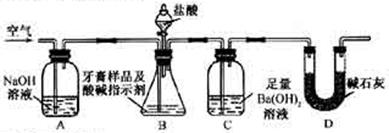
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 ��죬�ּ�����ʹ�����ǵ�ľ����ȼ������0.28L������£���
��죬�ּ�����ʹ�����ǵ�ľ����ȼ������0.28L������£����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
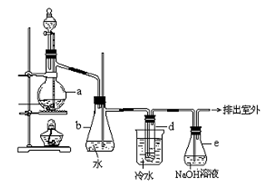
 ��ijƷ��������Ħ�����ɷּ��京����������̽����
��ijƷ��������Ħ�����ɷּ��京����������̽���� __________________________________________��
__________________________________________��
 ___��
___�� ��Ƶ���������Ϊ__________��
��Ƶ���������Ϊ__________���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| �ζ����� | ������Һ�����/mL | ����Һ����� | |
| �ζ�ǰ�̶�/mL | �ζ���̶�/mL | ||
| 1 | 25��00 | 1��02 | 21��03 |
| 2 | 25��00 | 2��00 | 21��99 |
| 3 | 25��00 | 0��20 | 20��20 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
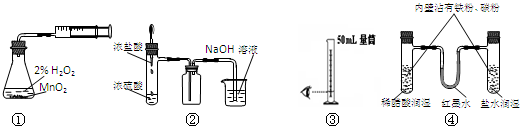
| A��װ�âٲⶨ��ѧ��Ӧ���� | B��װ�â���ȡ������HCl |
| C��װ�â���ȡ8.5mL��ϡ���� | D��װ�â�ģ�����ĸ�ʴ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����ȡ12.5g������CuSO4��5 H2O��������500mLˮ�����Һ |
| B����ȡ12.0g������CuSO4��5 H2O�������500mL��Һ |
| C����ȡ1.0g CuSO4�����500mL��Һ |
| D����ȡ12.5g������CuSO4��5 H2O�������500mL��Һ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com