��2010?˳����һģ������һ����Ҫ�Ļ���ԭ�ϣ�ijѧϰС������ȡ������̽�������ʣ���ش�
��1��ʵ������ȡ�����Ļ�ѧ����ʽ��
2NH
4Cl+Ca��OH��
2CaCl
2+2NH
3��+2H
2O
2NH
4Cl+Ca��OH��
2CaCl
2+2NH
3��+2H
2O
��
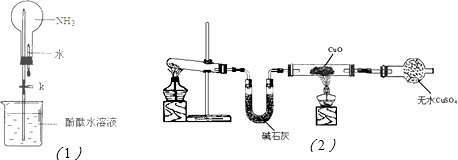
��2������ͼ��1���ǽ��а�����Ȫʵ���װ�ã�������Ȫ�IJ���������
���ἷѹ�ιܣ�ʹ����ˮ������ƿ��Ȼ���ֹˮ��K
���ἷѹ�ιܣ�ʹ����ˮ������ƿ��Ȼ���ֹˮ��K
��
�ڰ���ʹ�ձ�����Һ����ɫ��Ϊ��ɫ����ԭ����
NH3+H2O?NH3?H2O?NH4++OH-
NH3+H2O?NH3?H2O?NH4++OH-
���õ��뷽��ʽ��ʾ����
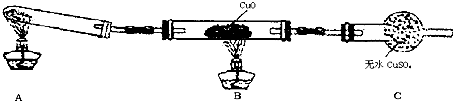
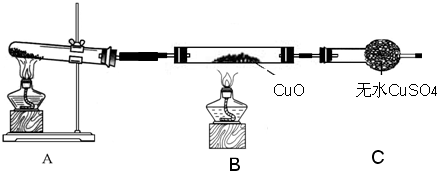
��3����С��ͬѧ�������ͼ��2����ʾ��ʵ��װ�ã����ּг�����δ��������̽�������Ļ�ԭ�Բ�������
��ʵ������Ϊ����ɫCuO��Ϊ��ɫ����ɫ��ˮCuSO
4��ĩ��Ϊ��ɫ��ͬʱ����һ����ɫ���壬����������Ⱦ����д��������CuO��Ӧ�Ļ�ѧ����ʽ
��
�ڼ�ʯ�ҵ�������
���հ����л��е�ˮ��������ֹ���Ų���ˮ�IJⶨ
���հ����л��е�ˮ��������ֹ���Ų���ˮ�IJⶨ
��
�۸�װ�ô�������ȱ�ݣ���ָ�����ڵ����Ⲣ����Ľ����
��װ������β������װ�ã�NH
3�����������Ⱦ����������һ�����հ���װ����ͼ��ʾ��

��
��װ������β������װ�ã�NH
3�����������Ⱦ����������һ�����հ���װ����ͼ��ʾ��

��
��
��4����ͬѧ��Ϊ��NH
3��CuO��Ӧ���ɵĺ�ɫ�����п��ܺ���Cu
2O����֪��Cu
2O��һ�ּ����������������Һ�У�Cu
+���ȶ��Ա�Cu
2+�Cu
+ Cu+Cu
2+�����������һ����ʵ�����ú�ɫ�������Ƿ���Cu
2O��
ȡ������Ʒ������ϡH2SO4������Һ������ɫ��˵����ɫ�����к���Cu2O��֮��û��
ȡ������Ʒ������ϡH2SO4������Һ������ɫ��˵����ɫ�����к���Cu2O��֮��û��
��
��5����ҵ�����еĵ�����������Ҫ�Ĵ�����Ⱦ��֮һ��Ϊ��������Ⱦ����ҵ�ϳ��ð�����֮������Ӧ��NO
x+NH
3��N
2+H
2O��ʹ��ת��Ϊ����N
2������NO
2��NO�Ļ������3.0L����3.4L��ͬ��ͬѹ�£�NH
3��Ӧ��ǡ��ʹ��ȫ��ת��Ϊ��������ԭ��������У�NO
2��NO���������
7��3
7��3
��

 ��Уͨ��֤��Ч��ҵϵ�д�
��Уͨ��֤��Ч��ҵϵ�д�

 ��
�� ��
��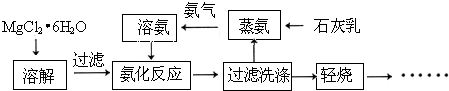
 2NH3��+CaCl2+2H2O
2NH3��+CaCl2+2H2O 2NH3��+CaCl2+2H2O
2NH3��+CaCl2+2H2O Mg2++2OH-�����Ȼ����Һ�д���NH4+��NH4++OH-
Mg2++2OH-�����Ȼ����Һ�д���NH4+��NH4++OH- NH3?H2O
NH3?H2O NH3��+H2O���μ�NH4Cl��Һ����ʹ���������ݳ�������ʹƽ�������ƶ�������Mg��OH��2�����ܽ�
NH3��+H2O���μ�NH4Cl��Һ����ʹ���������ݳ�������ʹƽ�������ƶ�������Mg��OH��2�����ܽ� Mg2++2OH-�����Ȼ����Һ�д���NH4+��NH4++OH-
Mg2++2OH-�����Ȼ����Һ�д���NH4+��NH4++OH- NH3?H2O
NH3?H2O NH3��+H2O���μ�NH4Cl��Һ����ʹ���������ݳ�������ʹƽ�������ƶ�������Mg��OH��2�����ܽ�
NH3��+H2O���μ�NH4Cl��Һ����ʹ���������ݳ�������ʹƽ�������ƶ�������Mg��OH��2�����ܽ�