
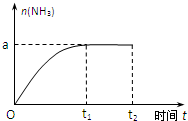
 ��֪N2��g��+3H2��g��?2NH3��g������H=-92.4kJ?mol-1��
��֪N2��g��+3H2��g��?2NH3��g������H=-92.4kJ?mol-1��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A������۵⻯����Һ�еμ�ϡ���ᣬ�ڿ����з���һ��ʱ�����Һ������4H++4I-+O2�T2I2+2H2O | ||||
| B����K2Cr2O7��Һ�еμ�����Ũ���ᣬ��Һ��Ϊ��ɫ��Cr2O72-����ɫ��+H2O?2CrO42-����ɫ��+2H+ | ||||
| C��0.01mol?L-1 NH4Al��SO4��2��Һ��0.02mol?L-1 Ba��OH��2��Һ���������а�ɫ�������ɣ�Al3++2SO42-+2Ba2++4OH-�T2BaSO4��+AlO2-+2H2O | ||||
D����ͭ�缫��ⱥ��ʳ��ˮ��2Cl-+2H2O
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| װ �� | �� �� |
 |
����ʵ���ʼ��δ���������� ������һ������������ݣ�Һ���Ϸ���dz��ɫ �����Թܱ��ȣ���Һ���� |
| ʵ �� | �� �� | �� �� |
| ʵ��1 | ��ʪ��KI-������ֽ���ڿ����� | δ���� |
| ʵ��2 | ��ʪ��KI-������ֽ����dz��ɫ���� | ��ֽ���� |
| װ �� | �� �� |
 |
����ʵ���ʼ��δ���������� ������һ������������ݣ��д̼�����ζ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ͨ����ˮ�ܻ�õ�ˮ��ʳ�Ρ�þ�ȣ�ʳ�οɽ�һ�������ȼҵ����ش��������⣮
ͨ����ˮ�ܻ�õ�ˮ��ʳ�Ρ�þ�ȣ�ʳ�οɽ�һ�������ȼҵ����ش��������⣮�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
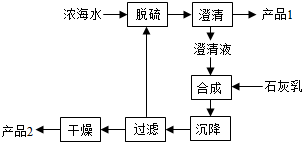
| ���� | Na+ | Mg2+ | Cl- | SO42- |
| Ũ��/��g?L-1�� | 63.7 | 28.8 | 144.6 | 46.4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ��� | A | B | C | D |
| ʵ�� ���� |
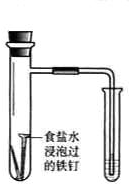 ʳ��ˮ |
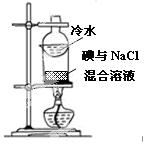 |
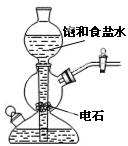 |
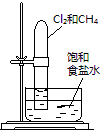 ���ڹ����� |
| ʵ�� Ŀ�� |
��֤�����������ⸯʴ | �ӵ���NaCl�����Һ�з������ | ʵ�����Ʊ���Ȳ | ��֤����������������ѧ��Ӧ |
| A��A | B��B | C��C | D��D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���÷�Ӧ��������ΪCuI����������ΪCu |
| B������Cu2HgI4�У�CuԪ�صĻ��ϼ�Ϊ+2 |
| C������2mol CuI���뷴Ӧʱ��ת�Ƶ���Ϊ1mol |
| D����Cu2+��Iֱ�ӷ�Ӧ�Ƶ�CuI�����ӷ���ʽΪCu2++I-=CuI |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����X��Y����ͬһ���ڣ���X��ԭ�Ӱ뾶�϶�С��Y��ԭ�Ӱ뾶 |
| B����X��Y���ڲ�ͬ�����ڣ���M����ˮ������Һ�϶��ʼ��� |
| C����M�������ӻ������û�������ֻ�������Ӽ� |
| D����M���ڹ��ۻ�̨���÷�����ԭ�Ӹ����ȿ���Ϊ1��2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A�����ø����������ҩ�ý��һ���˶Խ������Σ�� |
| B��ʳ��һ��������֬�ܴٽ������ijЩά���ص����� |
| C��CH4��Cl2�ڹ��������·�Ӧ�IJ��������������� |
| D��������ϩ�ļӳɣ�������ϩʹ����KMnO4��Һ��ɫ����������ں���̼̼˫���й� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com