
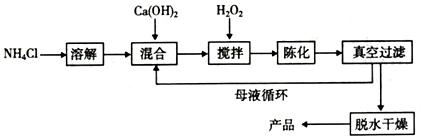
���� ��1��ʵ���Ŀ��Ϊ�Ʊ�CaO2•8H2O���������еij���ӦΪCaO2•8H2O�����������غ��жϻ�Ӧ��NH4Cl���ɣ����������غ㶨�ɿ�д����Ӧ�Ļ�ѧ����ʽ��CaO2•8H2O��0��ʱ�ȶ���Ϊ�˿��Ƴ����¶�Ϊ0�����ң���ʵ�����˲�ȡ�ķ����DZ�ˮԡ��ȴ���Դﵽʵ��Ŀ�ģ�
��2�����ݼ��Ƿ�Ӧ��Ҳ������������ʿ���ѭ�����ý��з�����
��3������ʵ��һ����2��CaO2•xH2O��$\frac{\underline{\;\;��\;\;}}{\;}$2CaO+O2��+2xH2O�и��ݵõ���O2�ڱ�״�������Ϊ134.4mL�������������������ʵ�����ʵ������������ɵ�̼������������ԭ��Ʒ�и�ԭ�ӵ����ʵ��������ԭ���غ�ȷ�������Ƶ�����������ȷ���������ƾ�����������Ӷ������ˮ�����������ʵ��������������������ˮ�����ʵ���֮�ȣ�ȷ��x��ֵ��
��� �⣺��1����ʵ���Ŀ��Ϊ�Ʊ�CaO2•8H2O���������еij���ӦΪCaO2•8H2O�����������غ��жϻ�Ӧ��NH4Cl���ɣ��ʿ�д����Ӧ�Ļ�ѧ����ʽΪ��CaCl2+H2O2+2NH3+8H2O=CaO2•8H2O��+2NH4Cl����CaCl2+H2O2+2NH3•H2O+6H2O=CaO2•8H2O��+2NH4Cl��CaO2•8H2O��0��ʱ�ȶ���Ϊ�˿��Ƴ����¶�Ϊ0�����ң���ʵ�����˲�ȡ�ķ����DZ�ˮԡ��ȴ���Դﵽʵ��Ŀ�ģ��ʴ�Ϊ��CaCl2+H2O2+2NH3+8H2O=CaO2•8H2O��+2NH4Cl����CaCl2+H2O2+2NH3•H2O+6H2O=CaO2•8H2O��+2NH4Cl����ˮԡ��ȴ����Ӧ���������ڱ�ˮ�У���
��2���Ȼ�識ȳ������˷�Ӧ���У�Ҳ���������������У������Ʊ������г�ˮ���ѭ��ʹ�õ������ǣ�NH4C1��
��3��O2�ڱ�״�������Ϊ 134.4mL�������ʵ���Ϊ$\frac{0.1344L}{22.4L/mol}$=0.006 mol������2��CaO2•xH2O��$\frac{\underline{\;\;��\;\;}}{\;}$2CaO+O2��+2xH2O��֪�������ƾ�������ʵ���Ϊ0.006 mol��2=0.012mol���������Ƶ����ʵ���0.012mol������Ϊ0.012mol��72g/mol=0.864g��̼��Ƴ��� 1.40g�����ʵ���Ϊ$\frac{1.40g}{100g/mol}$=0.014mol������ԭ���غ㣬ԭ��Ʒ��CaO�����ʵ���Ϊ0.014mol-0.012mol=0.002mol������Ϊ0.002mol��56g/mol=0.112g���������ƾ�����ˮ������Ϊ1.408g-0.112g-0.864g=0.432g��ˮ�����ʵ���Ϊ$\frac{0.432g}{18g/mol}$=0.024mol����x=$\frac{0.024mol}{0.012mol}$=2���ʴ�Ϊ��2��
���� ���⿼����ʽΪ�����Ʊ�����ͼ��Ŀ���漰���ʵĻ�ѧ����ʽ����д��ʵ�鷽����ʵ������ͼ�������⣬����ʱע��������عؼ���Ϣ������ʵ��������������⣬�����Ϊ�ۺϣ�


 ������ѧ���̲���ȫ���ϵ�д�
������ѧ���̲���ȫ���ϵ�д� ������ʱ����ҵ����ϵ�д�
������ʱ����ҵ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | �ٺ͢ڻ�Ϊͬ���칹�壬�ٺܻ͢�Ϊͬϵ�� | |
| B�� | �ܵ�һ�ȴ��������� | |
| C�� | �ڲ�������ϩ���������ӳɶ���� | |
| D�� | ��ͨ�����ⷴӦ�õ��۵�Ȳ����2�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��״���£�5.6L������̼�����к��е���ԭ����Ϊ0.5NA | |
| B�� | 0.1L0.5mol/LCH3COOH��Һ�к��е���������Ϊ0.05NA | |
| C�� | 1molFe���ڹ������ᣬ����ת����Ϊ2NA | |
| D�� | ��״���£�2.24LCC14���еĹ��ۼ���Ϊ0.4NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
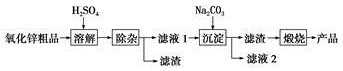
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
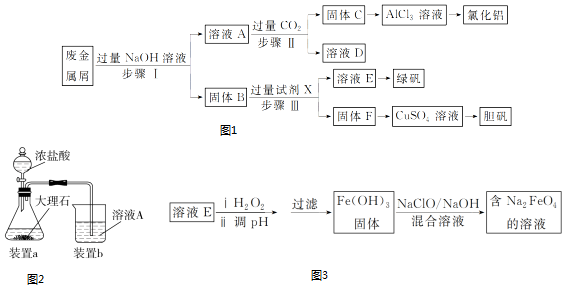
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
 �ж���ͬ���칹�壬�������������Һ��б����ṹ�Ĺ��У�������
�ж���ͬ���칹�壬�������������Һ��б����ṹ�Ĺ��У�������| A�� | 7�� | B�� | 6�� | C�� | 5�� | D�� | 4�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | NH3•H2O | B�� | BaSO4 | C�� | ���� | D�� | ϡ���� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com