

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����ȥþ���л��е��������ۣ���������ռ���Һ��ַ�Ӧ�����ˡ�ϴ�ӡ����� |
| B����ȥNa2CO3�����е�NaHCO3�����������м��� |
| C����ȥFe3+�е�Al3+����������İ�ˮ��ַ�Ӧ������ |
| D����ȥFeCl3������Һ�е�FeCl2��ͨ��������Cl2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��

| A���Ȼ�����Һ��������Һϡ���� |
| B���Ȼ�����Һϡ������������Һ |
| C������������Һ��������Һϡ���� |
| D����������Һ���ᱵ��Һϡ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��CO��HCl | B��CH4��NH3 | C��CH4��HCl | D��H2��CO2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| A����ȡ��Һ | B�����ȷֽ� | C�������ᾧ | D����Һ E������ F�����ˡ�G�����ȣ��뽫�ᴿ������������ں�������ϡ� |
 _____
_____ ______
______ �鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
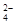 �IJ��������ǣ�
�IJ��������ǣ�  ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| A���ᾧ | B������ | C������ | D����Һ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com