
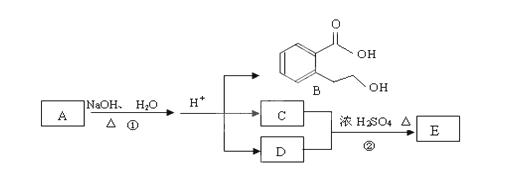
��ͼ�� A��B��C��D��E��Ϊ�л��������֪��C�ܸ�NaHCO3������Ӧ���ú�D����Է���������ȣ���EΪ��֧���Ļ����
������ͼ�ش����⣺
��1����֪E����Է�������Ϊ102������̼��������Ԫ�ص����������ֱ�Ϊ58.8%��9.8%������Ϊ����C�����еĹ����������� ______________��������B���������ķ�Ӧ�ǣ�����ĸ��ţ���______________
a���ӳɷ�Ӧ b��ȡ����Ӧ c����ȥ��Ӧ
d��������Ӧ e��ˮ�ⷴӦ f���û���Ӧ
��2����Ӧ�ڵĻ�ѧ����ʽ��__________________��
��3����Ӧ��ʵ���м��ȵ�Ŀ���ǣ� .��
��4��A�Ľṹ��ʽ�� __________________��
��5��д��ͬʱ������������������B��ͬ���칹������ͬ���칹��Ľṹ��ʽ��
���м��ȡ�������ṹ
�����ڷ������γɵ���
���� FeCl3��Һ������ɫ��Ӧ
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
8g��ˮ����ͭ���0.1mol/L������ͭˮ��Һ������˵����ȷ����
A������500mLˮ�� B������1Lˮ��
C���ܽ����Һ�������Ϊ500ml D���ܽ����Һ�������Ϊ1L
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ʵ���ܻ�óɹ����� (�� ��)
A������ȩ����������Һ�У��������������
B������Ũ��ˮ��Ӧ��ȡ�屽
C�����м�Ũ��ˮ�۲����
D��1 mol·L��1 CuSO4��Һ2 mL��0.5 mol·L��1 NaOH��Һ4 mL��Ϻ����40%����ȩ��Һ0.5 mL��������й۲��������ɫ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��NAΪ�����ӵ�����������˵������ȷ����
A��O2��O3�Ļ�����干6.4g������������ԭ����һ��Ϊ0.4NA
B������״����22.4LNO��11.2LO2��Ϻ�����ԼΪ22.4L
C���ڷ�ӦKClO4+8HCl=KCl+4Cl2��+4H2O�У�ÿ����4molCl2ת�Ƶĵ�����Ϊ8NA
D����״����11.2LCl2����1Lˮ�У�ת�Ƶ��ӵ���ĿΪ0.5NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
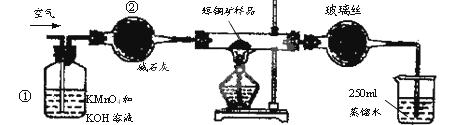
��ҵ��Ϊ�˲ⶨ��ͭ����Ҫ�ɷ���Cu2S����Cu2S�������������������ͼװ�á�ʵ��ʱ�����²��������
A.���Ӻ�������ʹ���Ϊ��ͼװ�ã������װ�õ������ԡ�
B.��ȡ��ϸ�Ļ�ͭ����Ʒ1.000g��
C.�������õ���ƷС�ĵط���Ӳ�ʲ������С�
D.��ÿ����1L�����ʹ��������
E.��Ӳ�ʲ������еĻ�ͭ����Ʒ���ȵ�һ���¶�,������ӦΪ:Cu2S+O2 SO2 +2Cu��
SO2 +2Cu��
F.��ȡ25.00mL��SO2��ˮ��Һ��250mL��ƿ�У���0.0100mol/L KMnO4����Һ�ζ����յ㡣���������������ظ��ζ�2��3�Ρ�
�Իش��������⣺
��1��װ�âٵ�������_________________��װ�âڵ�������____________________��
��2���ٶ���ͭ���е���ȫ��ת��ΪSO2������ȫ����ˮ���գ������F����������Ӧ�Ļ�ѧ����ʽΪ ��������_______________________________������ʱ���жϵζ��Ѿ��ﵽ�յ㡣
��3��������F�ĵζ�������±���ʾ�����ͭ����Ʒ��Cu2S������������________��
| �ζ� ���� | ������Һ��[��Դ:Z.X.X.K] ���/mL | ����Һ����� | |
| �ζ�ǰ�̶�/mL | �ζ���̶�/mL | ||
| 1 | 25.00 | 1.04 | 21.03 |
| 2 | 25.00 | 1.98 | 21.99 |
| 3 | 25.00 | 3.20 | 21.24 |
��4���������������һ�����Ե�ȱ��Ӱ���˲ⶨ����������ڲ���ʧ������Ϊ�� ��дһ�ּ��ɣ���
��5����֪�ڳ�����FeS �� Ksp�� 6 . 25 �� 10 ��18, H2S ������Һ�� c (H������ c (S2����֮��������¹�ϵ��c2 (H��)����S2��) = 1 . 0��10��22 ���ڸ��¶��£������� FeS Ͷ�����ⱥ����Һ�У���ʹ��Һ�У�Fe2+��Ϊ1 mol/L��Ӧ������Һ��c��Hʮ��Ϊ__________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵���У���������A��Ԫ�������������ǣ� ��
A�����ϵ���ԭ�Ӱ뾶��С B�����γ�-1������
C������������ˮ���������� D�����ϵ����⻯����ȶ������μ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ԫ�������ɷ���������˵���������(����)
A��Ra�ǵ������ڵڢ�A���Ԫ�أ�Ra(OH)2�ļ��Ա�Mg(OH)2�ļ���ǿ
B��As�ǵ������ڵڢ�A���Ԫ�أ�AsH3�Ļ�ԭ�Ա�NH3�Ļ�ԭ����
C��Cs��ԭ�Ӱ뾶��Na��ԭ�Ӱ뾶��Cs��ˮ��Ӧ��Na��ˮ��Ӧ������
D��Cl�ĺ˵������Al�ĺ˵������Cl��ԭ�Ӱ뾶��Al��ԭ�Ӱ뾶С
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
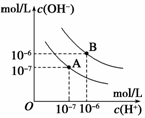
��֪ˮ��25���95��ʱ�������ƽ��������ͼ��ʾ����
��˵�������
A��A���ߴ���25��ʱˮ�ĵ���ƽ������
B����95��ʱ��pH=6����Һ������
C��25��ʱ����10mLpH=12��NaOH��Һ��1mLpH=1��
H2SO4 ��Һ��ϣ�������Һ��pH=7
D��95��ʱ������������ʵ���Ũ�ȵ�HA��Һ��NaOH��Һ��Ϻ������Һ��pH=6ʱ��˵��HA��Ϊ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������ͼ��ʾ��������ͨ�����û����ֿ����������Ҹ�����һ����NO��O2����ǡ��ʹ�������������ܶ���ͬ��������ʹNO��O2��ַ�Ӧ�����������ڻ�������ܶȱ�ԭ��
A.���� B. ��С
C.���� D. ��ȷ��

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com