
| |||||||||||||||||||||||||
(1) |
ֻ����ϩ���Ȼ���ļӳɷ�Ӧ���ܵõ��ϴ�����һ�����飬����ΪD�������������Ӧ�IJ���Ϊ�����ȴ�����Ļ�����ϩ�������ӳɷ�Ӧ�IJ���Ϊ1��2���������飻�������Ȼ��ⲻ��Ӧ����ϩ���Ȼ��ⷢ���ӳɷ�Ӧ�IJ���ΪCH3CH2Cl�� |
(2) |
�����𰸣�CH2 �����������ӳɷ�Ӧ�����ڲ�����̼ԭ���ϣ����Ի�ѧ����ʽ��CH2 |
(3) |
�����𰸣�һ������ķе�Ϊ12.27�棬������������������ʹ�ֲ��䶳������ ����������������ķе�ϵͣ�������������ʱ����������ʹ�ֲ��䶳���� |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
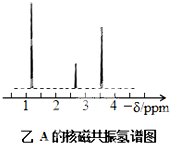 Ϊ�ⶨij�л�������A�Ľṹ����������ʵ�飮
Ϊ�ⶨij�л�������A�Ľṹ����������ʵ�飮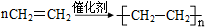
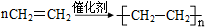
| ���� |
| ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
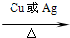 2CH3CHO+2H2O
2CH3CHO+2H2O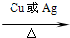 2CH3CHO+2H2O
2CH3CHO+2H2O| ˮԡ���� |
| ˮԡ���� |
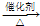
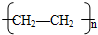 ��
��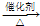
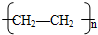 ��
��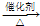 CH3CH2Cl��
CH3CH2Cl��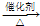 CH3CH2Cl��
CH3CH2Cl�� CH3COOC2H5+H2O��
CH3COOC2H5+H2O�� CH3COOC2H5+H2O��
CH3COOC2H5+H2O���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ���� |
| ���� |
| ||
| �� |
| ||
| �� |
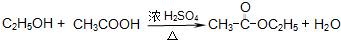
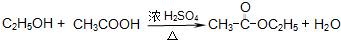
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ���� |
| �� |
| ���� |
| �� |
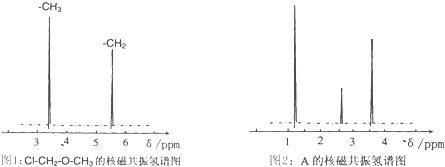
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com