
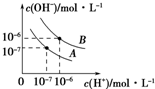
 ��֪ˮ��25���95��ʱ�������ƽ��������ͼ��ʾ��
��֪ˮ��25���95��ʱ�������ƽ��������ͼ��ʾ������ ��1��ˮ�ĵ��������ȹ��̣������¶ȴٽ�ˮ���룬����ˮ��c��H+����c��OH-��������
��2��ˮ�������ӡ���������������Ũ��Խ��ˮ�ĵ���̶�ԽС��ǡ�÷�Ӧ����Һ��ʾ���Ի���ԣ�
��3������ͼ���жϸ��¶���ˮ�����ӻ���Ȼ���������Һ������������Ũ�ȣ��ٽ�ϸ��¶���ˮ�����ӻ������������Һ��������Ũ�ȼ���Һ��pH��
��� �⣺��1��ˮ�ĵ��������ȹ��̣������¶ȣ�ʹˮ�ĵ���̶������¶�����ʱ���ٽ�ˮ�ĵ��룬ˮ�����ӻ�����ˮ�������ӡ�����������Ũ�ȶ�����ˮ��pH��С������Һ��Ȼ�����ԣ��ʴ�Ϊ��B��ˮ������Ҫ���ȣ��¶�Խ��KWԽ��
��2��������A�У�ˮ�����ӻ�Ϊ10-7��10-7=10-14��pH=2��HCl��c��H+��=10-2mol/L��pH=12��ijBOH��Һ��c��OH-��=10-2mol/L����c��H+��=c��OH-�����ʣ���1=��2��pH=2��HCl��c��H+��=10-2mol/L��pH=12��ijBOH��Һ��c��OH-��=10-2mol/L�����BOH��ǿ�����ߵ������ϣ���ʱǡ����ȫ��Ӧ������pH=7������BOH�������BOH��Ũ�Ȼ����0.01mol/L����ʱ����BCl��ˮ������ԣ�ʣ��IJ��ּ����ʼ��ԣ����߲�֪����˭Ϊ����������ȷ������ԣ�
�ʴ�Ϊ�����ڣ����жϣ�
��3��������B�У�ˮ�����ӻ�Ϊ��10-6��10-6=10-12��0.02mol/L��Ba��OH��2��Һ��c��OH-��=0.04mol/L��NaHSO4��Һ��Ũ��Ϊ0.02mol/L��c��H+��=0.02mol/L�������ߵ������Ϻ���Һ�е�c��OH-��=$\frac{0.04mol/L-0.02mol/L}{2}$=0.01mol/L����c��H+��=$\frac{1{0}^{-12}}{0.01}$=10-10mol/L������Һ��pH=10���ʴ�Ϊ��10��
���� ���⿼��ˮ�ĵ�����ᡢ���ϵ�pH�������⣬�ѶȽϴ�ע���¶Ȳ�ͬ��ʹˮ�����ӻ�������ͬ�ǽⱾ��Ĺؼ�����3��Ϊ�״��㣬��Ҫ��ȷ���¶���ˮ�����ӻ�Ϊ10-6��10-6=10-12�������ֿ�����ѧ���ķ�����������������ѧ����������


 ��ѧ����ϵ�д�
��ѧ����ϵ�д� �ο�������ϵ�д�
�ο�������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
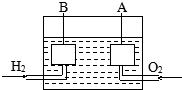 ����ɴ���ʹ�õ�����ȼ�ϵ����һ�����ͻ�ѧ��أ��乹����ͼ��ʾ��A��B�Ƕ����̿�Ƴɵ������缫��ͨ��������ɿ�϶���ݳ������ڵ缫����ŵ磮
����ɴ���ʹ�õ�����ȼ�ϵ����һ�����ͻ�ѧ��أ��乹����ͼ��ʾ��A��B�Ƕ����̿�Ƴɵ������缫��ͨ��������ɿ�϶���ݳ������ڵ缫����ŵ磮�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 0.5mol��K+ | B�� | 0.5mol��H+ | C�� | 1.0mol��H+ | D�� | 1.0mol��OH- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����Һ�����Ȼ�����Һ�а�ɫ�������ɣ��ټ������������ʧ�Ҳ�����ɫ��ζ�����壬�����Һ��һ������CO32- | |
| B�� | �����Ȼ�����Һ�а�ɫ�������ɣ��ټ�ϡ���ᣬ��������ʧ��һ������SO42- | |
| C�� | ��������ij�����е�ȼ����������ɫ���棬�������Ϊ���� | |
| D�� | ����Һ����NaOH��Һ�����ȣ�����ʹʪ��ĺ�ɫʯ����ֽ�������������ɣ���ԭ��Һ��һ������NH4+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���� | B�� | �� | C�� | ���Ȼ�̼ | D�� | �ƾ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������Һ�����Ϊ��m-n��mL | B�� | ������Һ�����Ϊ��n-m��mL | ||
| C�� | ������Һ������ڣ�a-n��mL | D�� | ������Һ�������nmL |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com