
N2O5��һ����������������һ���¶��¿ɷ������з�Ӧ�� ![]() ��H >0
��H >0
T1�¶��µIJ���ʵ������Ϊ��
| t/s | 0 | 500 | 1000 | 1500 |
| c��N2O5��mol/L | 5.00 | 3.52 | 2.50 | 2.50 |
����˵������ȷ���� �� ��
A��500s��N2O5�ֽ�����Ϊ2.96��10��3 mol/(L�� s)
B��T1�¶��µ�ƽ�ⳣ��ΪK1 =125��1000sʱת����Ϊ50%
C��������������ʱ��T2�¶��·�Ӧ��1000sʱ���N2O5��g��Ũ��Ϊ2.98 mol/L����T1 <T2
D��T1�¶��µ�ƽ�ⳣ��ΪK1 ��T3�¶��µ�ƽ�ⳣ��ΪK3����K1> K3����T1 >T3
 �����ҵ��ٿ���������������ϵ�д�
�����ҵ��ٿ���������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2008?���죩N2O5��һ�������������������ʺ��Ʊ��ܵ����ǵĹ�ע��
��2008?���죩N2O5��һ�������������������ʺ��Ʊ��ܵ����ǵĹ�ע��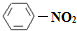
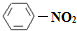
| t/s | 0 | 500 | 1000 |
| e��N2O5��/mol?L-1 | 5.00 | 3.52 | 2.48 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ��X��Y��Z�Ƕ�����Ԫ�ص����ֳ��������X��ˮ��Ӧ������һ�־��л�ԭ�ԵIJ��ȶ��Ķ�Ԫ�ᣬ����Ļ�ѧʽ��
��X��Y��Z�Ƕ�����Ԫ�ص����ֳ��������X��ˮ��Ӧ������һ�־��л�ԭ�ԵIJ��ȶ��Ķ�Ԫ�ᣬ����Ļ�ѧʽ��
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
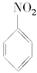
��1��N2O5�뱽����������Ӧ���ɵ��������Ľṹ��ʽ��____________________________��
��2��һ���¶��£��ں����ܱ�������N2O5�ɷ������з�Ӧ��
2N2O5��g��![]() 4NO2��g��+O2��g������H��0
4NO2��g��+O2��g������H��0
�ٷ�Ӧ��ƽ�������ͨ��һ������������N2O5��ת���ʽ�___________�������������С�����䡱��
���±�Ϊ��Ӧ��T1�¶��µIJ���ʵ�����ݣ�
t/s | 0 | 500 | 1 000 |
c��N2O5��/mol��L-1 | 5.00 | 3.52 | 2.48 |
��500 s��N2O5�ķֽ�����Ϊ__________��
����T2�¶��£���Ӧ1 000 sʱ���NO2��Ũ��Ϊ4.98 mol��L-1����T2___________T1��
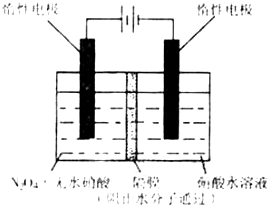
��3����ͼ��ʾװ�ÿ������Ʊ�N2O5����N2O5�ڵ��ص�_______�����ɣ���缫��ӦʽΪ____________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
N2O5��һ�������������������ʺ��Ʊ��ܵ����ǵĹ�ע��![]()
![]()
![]()
![]()
![]()
![]()
![]()
��1��N2O5�뱽����������Ӧ���ɵ��������Ľṹ��ʽ�� ��
��2��һ���¶��£��ں����ܱ�������N2 O5�ɷ������з�Ӧ��
![]() 2N2O5��g�� 4NO2��g��+O2(g);��H��0
2N2O5��g�� 4NO2��g��+O2(g);��H��0
�ٷ�Ӧ��ƽ�������ͨ��һ������������N2O5��ת���ʽ� ���������С���������䡱��
���±�Ϊ��Ӧ��T1�¶��µIJ���ʵ�����ݣ�
| t/s | 0 | 500 | 1000 |
| c(N2O5)/mol��L-1 | 5.00 | 3.52 | 2.48 |
��500s��N2O5�ķֽ�����Ϊ ��
����T3�¶��£���Ӧ1 000 sʱ���NO2��Ũ��Ϊ4.98 mol��L-1����T2 T1��
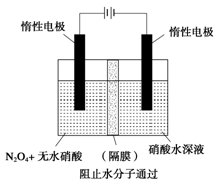 |
��3������ͼ��ʾװ�ÿ������Ʊ�N2O5����N2O5�ڵ��ص�
�����ɣ���缫��ӦʽΪ .
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��.��˹�����������Ϳ�ѧ�о����к���Ҫ�����塣��Щ��Ӧ�ķ�Ӧ����Ȼ��ֱ�Ӳ�ã�����ͨ����ӵķ����ⶨ���ָ������е�5����Ӧ(�ɰ�����HCl��ˮ�Ʊ�NH4C1ˮ��Һ)�����жϷ�Ӧ�ܵķ�Ӧ�ȣ���H�� ��
�� NH3(g) + HCl(g) = NH4Cl(s) ��H=��176kJ��mol�C1
�� NH3(g) + H2O(l) = NH3(aq) ��H=��35.1 kJ��mol�C1
�� HCl(g) + H2O(l) = HCl(aq) ��H=��72.3 kJ��mol�C1
�� NH4C1(s) + H2O(1) = NH4C1(aq)
�� NH3(aq) + HCl(aq) = NH4C1(aq) ��H= ��52.3 kJ��mol�C1
��. N2O5��һ�������������������ʺ��Ʊ��ܵ����ǵĹ�ע������H2��O2��������Na2CO3��ɵ�ȼ�ϵ�أ����õ�ⷨ�Ʊ�N2O5��װ����ͼ��ʾ������YΪCO2��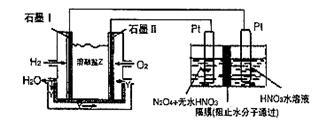
д��ʯīI�缫�Ϸ�����Ӧ�ĵ缫��Ӧʽ ��
�ڵ���������N2O3�ĵ缫��ӦʽΪ ��
��.����������SO2����������������NOx���������ǻ�����ѧ�о����ȵ㡣
���������������Ļ��������� ��
(2)Ŀǰ����ѧ�������о�һ������ϩ��Ϊ��ԭ����������NO��ԭ��������������ʾ��ͼ����ͼ1�����������¶ȡ������ʣ�����ɸ�д����������������Ĺ�ϵ��ͼ2��ʾ��
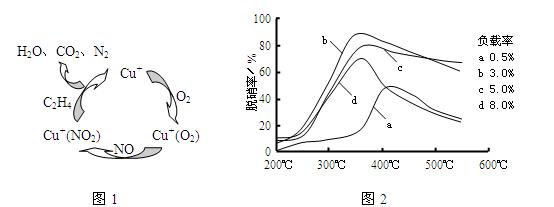
��д��������ԭ���ܷ�Ӧ�Ļ�ѧ����ʽ�� ����Ϊ�ﵽ�������Ч����Ӧ��ȡ�������� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com