
 �����õ���ʽ��ʾA��C�γɻ�����Ĺ���
�����õ���ʽ��ʾA��C�γɻ�����Ĺ���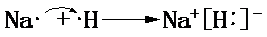 ��
������ A��B��C��D��E��FΪ����������Ԫ�أ���ԭ��������������A��ԭ�Ӱ뾶��С��Ԫ�أ���AΪHԪ�أ�B������������ˮ����������⻯�ﷴӦ�γ����ӻ�����ף���ΪBΪNԪ�أ���ΪNH4NO3��E��Bͬ���壬��EΪPԪ�أ�A��D������ԭ�Ӹ�����4��1�γɻ������ң����ҷ����к���18�����ӣ���DΪSi����ΪSiH4�����ԭ��������֪��C��F�����ڵ������ڣ�Fԭ�����������ף�C����������F�����������һ�����Ӳ㣬�ҿ��γ������ӡ������Ӹ�����Ϊ2��1�����ӻ����������FΪS��CΪNa����ΪNa2S���ݴ˽��
��� �⣺A��B��C��D��E��FΪ����������Ԫ�أ���ԭ��������������A��ԭ�Ӱ뾶��С��Ԫ�أ���AΪHԪ�أ�B������������ˮ����������⻯�ﷴӦ�γ����ӻ�����ף���ΪBΪNԪ�أ���ΪNH4NO3��E��Bͬ���壬��EΪPԪ�أ�A��D������ԭ�Ӹ�����4��1�γɻ������ң����ҷ����к���18�����ӣ���DΪSi����ΪSiH4�����ԭ��������֪��C��F�����ڵ������ڣ�Fԭ�����������ף�C����������F�����������һ�����Ӳ㣬�ҿ��γ������ӡ������Ӹ�����Ϊ2��1�����ӻ����������FΪS��CΪNa����ΪNa2S��
��1��B����̬�⻯��ΪNH3������ʽΪ ��A��C�γɻ�����ΪNaH���õ���ʽ��ʾ���γɹ���Ϊ��
��A��C�γɻ�����ΪNaH���õ���ʽ��ʾ���γɹ���Ϊ��
�ʴ�Ϊ�� ��
��
��2��EΪPԪ�أ������ڱ��е�λ��Ϊ���������ڵ�VA�壬
�ʴ�Ϊ���������ڵ�VA�壻
��3���ٻ�������ΪSiH4��������ֻ���м��Թ��ۼ����ʢ���ȷ��
�ڻ�����ΪNH4NO3���������Ӽ������ۼ����������ΪNa2S��ֻ�������Ӽ����ʢڴ���
�۷ǽ�����B��N����E��P�������⻯����B�ĸ��ȶ����ʢ���ȷ��
��ͬ�����������ԭ�Ӱ뾶��С����ԭ�Ӱ뾶C��Na����D��Si����E��P����F��S�����ʢ���ȷ��
��ѡ���٢ۢܣ�
��4��������Ԫ�ع��ɵ�10���ӷ�����18���ӷ��Ӱ����ʵ���֮��1��1��Ӧ�����Σ�Ӧ�ǰ��������ⷴӦ�������⻯泥���Ӧ��ѧ����ʽΪ��NH3+H2S=NH4HS��
�ʴ�Ϊ��NH3+H2S=NH4HS��
���� ���⿼��λ�ýṹ�����ʹ�ϵ���ۺ�Ӧ�ã��漰���û�ѧ���Ԫ�������ɡ�Ԫ�ػ��������ʵȣ�ע��Ի���֪ʶ���������գ�


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� |  ��ͼ��������к�����ϩ | B�� |  ��ͼ��ȥCO�л��е�����CO2 | ||
| C�� |  ��ͼ��֤��ѹ����ԭ�� | D�� |  ��ͼ�ռ�NH3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | Na+��SO42-��Cu2+��Cl- | B�� | Fe2+��Na+��OH-��K+ | ||
| C�� | K+��CO32-��Cl-��Ag+ | D�� | H+��Cl-��Na+��CO32- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���� | B�� | ��NaOH | C�� | ͨCO2���� | D�� | ������ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com