
(10·Ö)ČēĶ¼ĖłŹ¾µÄŹµŃé×°ÖĆÖŠ£¬EĪŖŅ»ÕÅÓƵķ·Ū”¢µā»Æ¼ŲŗĶ·ÓĢŖ»ģŗĻČÜŅŗČóŹŖµÄĀĖÖ½£¬C”¢DĪŖ¼ŠŌŚĀĖÖ½Į½¶ĖµÄ²¬¼Š£»x”¢y·Ö±šĪŖÖ±Į÷µēŌ“µÄĮ½¼«”£ŌŚA”¢BÖŠ³äĀśKOHČÜŅŗŗóµ¹Į¢ÓŚŹ¢ÓŠKOHČÜŅŗµÄĖ®²ŪÖŠ£¬ŌŁ·Ö±š²åČėŅ»¶ąæ׵ĶčŠŌµē¼«”£ĒŠ¶ĻµēŌ“æŖ¹ŲS£±£¬±ÕŗĻæŖ¹ŲS£²£¬ĶØÖ±Į÷µēŅ»¶ĪŹ±¼äŗó£¬Éś³ÉĘųĢåČēĶ¼ĖłŹ¾”£
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)±ź³öµēŌ“µÄÕż”¢øŗ¼«£ŗxĪŖ”””” ”£
(2)ŌŚĀĖÖ½µÄC¶Ėø½½ü£¬¹Ū²ģµ½µÄĻÖĻóŹĒ”” ”” ”” ”£?
(3)Š“³öµē¼«·“Ó¦Ź½£ŗBµē¼«”””””” ”””””””””””””””””” ”””””” ”£?
(4)Čōµē½āŅ»¶ĪŹ±¼äŗó£¬A”¢BÖŠ¾łÓŠĘųĢå°üĪ§µē¼«”£“ĖŹ±ĒŠ¶ĻæŖ¹ŲS£²±ÕŗĻæŖ¹ŲS£±£¬ŌņµēĮ÷¼ĘµÄÖøÕėŹĒ·ń·¢ÉśĘ«×Ŗ”””””””””” (Ģī”°Ę«×Ŗ”±»ņ”°²»Ę«×Ŗ”±)”£?
(5)ČōµēĮ÷¼ĘÖøÕėĘ«×Ŗ£¬Š“³öÓŠ¹ŲµÄµē¼«·“Ó¦(ČōÖøÕė”°²»Ę«×Ŗ”±£¬“ĖĢā²»±Ų»Ų“š”£)£»
”””””” ”””””””””” ”” ”””” ”””””””” ”£?
ČōµēĮ÷¼ĘÖøÕė²»Ę«×Ŗ£¬ĒėĖµĆ÷ĄķÓÉ(ČōÖøÕė”°Ę«×Ŗ”±£¬“ĖĢā²»±Ų»Ų“š)”””””” ”£
(10·Ö)¢Å Õż £Ø1·Ö£© ¢Ę ĀĖÖ½±äĄ¶ £Ø2·Ö£© ¢Ē B¼«£ŗ4OHØDØD4e=2H2O+O2”ü £Ø2·Ö£©
¢Č Ę«×Ŗ £Ø1·Ö£© ¢É 2H2+4OHØDØD4e=4H2O £Ø2·Ö£© 2H2O+O2+4e=4OH£ £Ø2·Ö£©
½āĪöŹŌĢā·ÖĪö£ŗ£Ø1£©¶čŠŌµē¼«µē½āKOHČÜŅŗ£¬ŹµÖŹ¾ĶŹĒµē½āĖ®£¬ĖłµĆ²śĪļĪŖH2ŗĶO2”£ŅņĪŖAŹŌ¹ÜÖŠĘųĢåĢå»żŹĒBŹŌ¹ÜÖŠµÄ2±¶£¬ĖłŅŌAÖŠĪŖH2£¬BÖŠĪŖO2£¬¼“AĪŖŅõ¼«£¬BĪŖŃō¼«£¬ĖłŅŌXĪŖÕż¼«£¬YĪŖøŗ¼«”£
£Ø2£©CÓėµēŌ“Õż¼«ĻąĮ¬£¬ĪŖŃō¼«£¬µē¼«·“Ó¦·½³ĢŹ½ĪŖ2I££2e££½I2£¬µāÓöµķ·ŪĻŌĄ¶É«£¬ĖłŅŌŹŌÖ½±äĄ¶”£
£Ø3£©Bµē¼«ŹĒŃō¼«£¬ČÜŅŗÖŠµÄOH£·Åµē£¬µē¼«·“Ó¦Ź½ŹĒ4OHØDØD4e££½2H2O+O2”ü”£
£Ø4£©Čē¹ūĒŠ¶ĻS2£¬±ÕŗĻS1£¬ŌņæÉÓÉAÖŠµÄH2£¬BÖŠµÄO2ÓėKOHČÜŅŗŠĪ³ÉH2£O2Č¼ĮĻµē³Ų£¬°Ń»ÆѧÄܱäĪŖµēÄÜ£¬Ņņ“ĖÖøÕėĘ«×Ŗ”£
£Ø5£©ŌŚŌµē³ŲÖŠøŗ¼«Ź§Č„µē×Ó£¬Õż¼«µĆµ½µē×Ó£¬ĖłŅŌĒāĘųŅ»¼«ŹĒøŗ¼«£¬ŃõĘųŅ»¼«ŹĒÕż¼«£¬µē¼«·“Ó¦Ź½·Ö±šŹĒ£ŗA¼« 2H2£«4OH££4e££½4H2O”¢B¼« 2H2O£«O2£«4e££½4OH£”£
æ¼µć£ŗæ¼²éµē¼«Ćū³Ę”¢µē¼«·“Ó¦Ź½µÄÅŠ¶ĻŗĶŹéŠ“
µćĘĄ£ŗøĆĢāŹĒøßæ¼ÖŠµÄ³£¼ūĢāŠĶŗĶÖŲŅŖµÄæ¼µćÖ®Ņ»£¬ŹōÓŚÖŠµČÄѶȵďŌĢā”£ŹŌĢā×ŪŗĻŠŌĒ棬ÄŃŅ׏ŹÖŠ£¬Ģł½üøßæ¼£¬ÓŠĄūÓŚÅąŃųѧɜ·ÖĪöĪŹĢā”¢½ā¾öĪŹĢāµÄÄÜĮ¦£¬Ņ²ÓŠÖśÓŚÅąŃųѧɜµÄĀß¼Ė¼Ī¬ÄÜĮ¦ŗĶ·¢É¢Ė¼Ī¬ÄÜĮ¦£¬ĢįøßѧɜѧĻ°Š§ĀŹ”£


 ĢģĢģĻņÉĻŅ»±¾ŗĆ¾ķĻµĮŠ“š°ø
ĢģĢģĻņÉĻŅ»±¾ŗĆ¾ķĻµĮŠ“š°ø Š”ѧɜ10·ÖÖÓÓ¦ÓĆĢāĻµĮŠ“š°ø
Š”ѧɜ10·ÖÖÓÓ¦ÓĆĢāĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2012½ģøßČżÉĻѧʌ»ÆѧŅ»ĀÖø“Ļ°”¶Ī¢¹Ū½į¹¹ÓėĪļÖŹµÄ¶ąŃłŠŌ”·×ØĢā×ŪŗĻ²āŹŌ£ØĖÕ½Ģ°ę£© ĢāŠĶ£ŗĢīæÕĢā
(8·Ö)ŅŃÖŖA”¢B”¢C”¢DŹĒ֊ѧ»Æѧ֊³£¼ūµÄĖÄÖÖ²»Ķ¬Ī¢Į£”£ĖüĆĒÖ®¼ä“ęŌŚČēĶ¼ĖłŹ¾µÄ×Ŗ»Æ¹ŲĻµ”£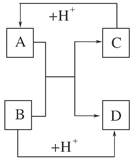
(1)Čē¹ūA”¢B”¢C”¢D¾łŹĒ10µē×ÓµÄĪ¢Į££¬ĒėŠ“³ö£ŗAµÄ½į¹¹Ź½________£¬DµÄµē×ÓŹ½________”£
(2)Čē¹ūAŗĶCŹĒ18µē×ÓµÄĪ¢Į££¬BŗĶDŹĒ10µē×ÓµÄĪ¢Į££¬ĒėŠ“³ö£ŗ
¢ŁAÓėBŌŚČÜŅŗÖŠ·“Ó¦µÄĄė×Ó·½³ĢŹ½___________________________________
________________________________________________________________________ӣ
¢Śøł¾ŻÉĻŹöĄė×Ó·½³ĢŹ½£¬æÉÅŠ¶ĻCÓėB½įŗĻÖŹ×ÓµÄÄÜĮ¦“󊔏Ē(ÓĆ»ÆѧŹ½»ņĄė×Ó·ūŗűķŹ¾)________>________”£
(3)ŅŃÖŖėĀ(H2N”ŖNH2)ŗĶ¼×°·(CH3”ŖNH2)¶¼ŹĒ18øöµē×ӵķÖ×Ó”£·ÖĪöėĀŗĶ¼×°·µÄ½į¹¹ĢŲµć²¢“ÓÖŠŹÜµ½Ęō·¢£¬Š“³öÓėĘä¾ßÓŠĻąĶ¬µē×ÓŹżµÄÓŠ»ś»ÆŗĻĪļµÄ½į¹¹¼ņŹ½(ÖĮÉŁŠ“Į½øö)£ŗ________________________________________________________________________
________________________________________________________________________ӣ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2012-2013ѧğŗÓ±±Ź”ŹÆ¼Ņ×ÆŹŠµŚŅ»ÖŠŃ§ø߶žÉĻŃ§ĘŚĘŚÖŠæ¼ŹŌ»ÆѧŹŌ¾ķ£Ø“ų½āĪö£© ĢāŠĶ£ŗŹµŃéĢā
)ŹµŃéŹŅÖʱøäå±½æÉÓĆČēĶ¼ĖłŹ¾µÄ×°ÖĆ£¬ĢīŠ“ĻĀĮŠæÕ°×£ŗ
£Ø1£©¹Ų±ÕF¼Š£¬“ņæŖC¼Š£¬ŌŚ×°ÓŠÉŁĮæ±½µÄČżæŚÉÕĘæÖŠÓÉAæŚ¼ÓČėÉŁĮæä壬ŌŁ¼ÓČėÉŁĮæĢśŠ¼£¬Čū×”
AæŚ£¬·“Ó¦Ņ»¶ĪŹ±¼äÖʵÄäå±½”£äå±½ŹĒŅ»ÖÖĆܶȱČĖ® (Ģī”±Š””±»ņ”±“ó”±)µÄĪŽÉ«ŅŗĢ壬ŌŚŹµ
ŃéÖŠŅņĪŖ ¶ųĻŌŗÖÉ«”£ŌņČżæŚÉÕĘæÖŠ·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ__ ___ŗĶ_ __”£
£Ø2£©D”¢EŹŌ¹ÜÄŚ³öĻÖµÄĻÖĻóĪŖ_____________________________________”£
£Ø3£©“żČżæŚÉÕĘæÖŠµÄ·“Ó¦½ųŠŠµ½ČŌÓŠĘųÅŻĆ°³öŹ±ĖÉæŖF¼Š£¬¹Ų±ÕC£¬æÉŅŌ擵½µÄĻÖĻóŹĒ
____________________________________ӣ
£Ø4£©ČżæŚÉÕĘæÖŠµÄäå±½¾¹żĻĀĮŠ²½Öč·ÖĄėĢį“æ£ŗ
¢ŁĻņČżæŚÉÕĘæÖŠ¼ÓČė10 mLĖ®£¬Č»ŗó¹żĀĖ³żČ„Ī“·“Ó¦µÄĢśŠ¼£»
¢ŚĀĖŅŗŅĄ“ĪÓĆ10 mLĖ®”¢8 mL10£„µÄNaOHČÜŅŗ”¢10 mLĖ®Ļ“µÓ”£NaOHČÜŅŗĻ“µÓµÄ×÷ÓĆŹĒ_______________________£»
¢ŪĻņ·Ö³öµÄ“Öäå±½ÖŠ¼ÓČėÉŁĮæµÄĪŽĖ®ĀČ»ÆøĘ£¬¾²ÖĆ”¢¹żĀĖ”£¼ÓČėĀČ»ÆøʵÄÄæµÄŹĒ_ __”£
(5) ¾¹żÉĻŹö·ÖĄė²Ł×÷ŗ󣬓Öäå±½ÖŠ»¹ŗ¬ÓŠµÄÖ÷ŅŖŌÓÖŹĪŖ_____________£¬ŅŖ½ųŅ»²½Ģį“棬ĻĀĮŠ²Ł
×÷ÖŠ±ŲŠėµÄŹĒ_______£ØĢīČėÕżČ·Ń”ĻīĒ°µÄ×ÖÄø£©£ŗ
A£®ÖŲ½į¾§ B£®¹żĀĖ C£®ÕōĮó D£®ŻĶČ”
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2011-2012ѧğøßČżÉĻѧʌ»ÆѧŅ»ĀÖø“Ļ°”¶Ī¢¹Ū½į¹¹ÓėĪļÖŹµÄ¶ąŃłŠŌ”·×ØĢā×ŪŗĻ²āŹŌ£ØĖÕ½Ģ°ę£© ĢāŠĶ£ŗĢīæÕĢā
(8·Ö)ŅŃÖŖA”¢B”¢C”¢DŹĒ֊ѧ»Æѧ֊³£¼ūµÄĖÄÖÖ²»Ķ¬Ī¢Į£”£ĖüĆĒÖ®¼ä“ęŌŚČēĶ¼ĖłŹ¾µÄ×Ŗ»Æ¹ŲĻµ”£

(1)Čē¹ūA”¢B”¢C”¢D¾łŹĒ10µē×ÓµÄĪ¢Į££¬ĒėŠ“³ö£ŗAµÄ½į¹¹Ź½________£¬DµÄµē×ÓŹ½________”£
(2)Čē¹ūAŗĶCŹĒ18µē×ÓµÄĪ¢Į££¬BŗĶDŹĒ10µē×ÓµÄĪ¢Į££¬ĒėŠ“³ö£ŗ
¢ŁAÓėBŌŚČÜŅŗÖŠ·“Ó¦µÄĄė×Ó·½³ĢŹ½___________________________________
________________________________________________________________________ӣ
¢Śøł¾ŻÉĻŹöĄė×Ó·½³ĢŹ½£¬æÉÅŠ¶ĻCÓėB½įŗĻÖŹ×ÓµÄÄÜĮ¦“󊔏Ē(ÓĆ»ÆѧŹ½»ņĄė×Ó·ūŗűķŹ¾)________>________”£
(3)ŅŃÖŖėĀ(H2N”ŖNH2)ŗĶ¼×°·(CH3”ŖNH2)¶¼ŹĒ18øöµē×ӵķÖ×Ó”£·ÖĪöėĀŗĶ¼×°·µÄ½į¹¹ĢŲµć²¢“ÓÖŠŹÜµ½Ęō·¢£¬Š“³öÓėĘä¾ßÓŠĻąĶ¬µē×ÓŹżµÄÓŠ»ś»ÆŗĻĪļµÄ½į¹¹¼ņŹ½(ÖĮÉŁŠ“Į½øö)£ŗ________________________________________________________________________
________________________________________________________________________ӣ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2013½ģŗžÄĻŹ”ø߶ž2ŌĀŌĀæ¼»Æѧ£ØĄķ£©ŹŌ¾ķ ĢāŠĶ£ŗŹµŃéĢā
£Ø14·Ö£©1£ŅŅŃõ»łŻĮŹĒŅ»ÖÖĪŽÉ«ŅŗĢ壬ĆܶȱČĖ®“󣬲»ČÜÓŚĖ®£¬Ņ×ČÜÓŚ“¼”¢ĆŃ£¬ČŪµć5.5 ”ę£¬·Šµć267.4 ”ę”£1£ŅŅŃõ»łŻĮ³£ÓĆ×÷ĻćĮĻ£¬Ņ²æÉ×÷ĪŖŗĻ³ÉĘäĖūĻćĮĻµÄŌĮĻ”£ŹµŃéŹŅÖʱø1£ŅŅŃõ»łŻĮµÄ¹ż³ĢČēĻĀ£ŗ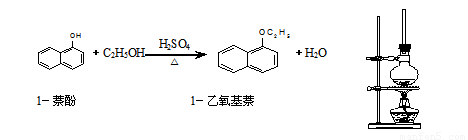
(1)½«»ģŗĻŅŗÖĆÓŚČēĶ¼ĖłŹ¾µÄČŻĘ÷ÖŠ¼ÓČČ³ä·Ö·“Ó¦”£ŹµŃéÖŠŹ¹ÓĆ¹ż
ĮæŅŅ“¼µÄŌŅņŹĒ ”£ÉÕĘæÉĻĮ¬½Ó³¤Ö±²£Į§¹ÜµÄÖ÷ŅŖ
×÷ÓĆŹĒ ”£
£Ø2£©·“Ó¦½įŹų£¬½«ÉÕĘæÖŠµÄŅŗĢåµ¹ČėĄäĖ®ÖŠ£¬¾“¦ĄķµĆµ½ÓŠ»ś²ć”£ĪŖĢį“æ²śĪļÓŠŅŌĻĀĖIJ½²Ł×÷£ŗ ¢ŁÕōĮó ¢ŚĖ®Ļ“²¢·ÖŅŗ ¢ŪÓĆ10%µÄNaOHČÜŅŗ¼īĻ“²¢·ÖŅŗ ¢ÜÓĆĪŽĖ®ĀČ»ÆøĘøÉŌļ²¢¹żĀĖ”£ÕżČ·µÄĖ³ŠņŹĒ ”£
A£®¢Ū¢Ś¢Ü¢Ł B£®¢Ł¢Ś¢Ū¢Ü C£®¢Ś¢Ł¢Ū¢Ü
£Ø3£©ÕōĮóŹ±ĖłÓĆµÄ²£Į§ŅĒĘ÷³żĮĖ¾Ę¾«µĘ”¢ĄäÄż¹Ü”¢½ÓŹÕĘ÷”¢
׶ŠĪĘæĶā»¹ÓŠ £¬ ”£
£Ø4£©ŹµŃé²āµĆ1£ŅŅŃõ»łŻĮµÄ²śĮæÓė·“Ó¦Ź±¼ä”¢ĪĀ¶ČµÄ±ä»Æ
ČēĶ¼ĖłŹ¾£¬Ź±¼äŃÓ³¤”¢ĪĀ¶ČÉżøß1£ŅŅŃõ»łŻĮµÄ²śĮæĻĀ
½µµÄŌŅņæÉÄÜŹĒ ”¢ ”£

£Ø5£©ÓĆ½šŹōÄĘæɼģŃé1£ŅŅŃõ»łŻĮŹĒ·ń“æ¾»£¬¼ņŹöŹµŃéĻÖĻóÓė½įĀŪ£ŗ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2010ÄźÉ³ŗÓŅ»ÖŠæ¼Ē°Ō¤²ā¾ķ»ÆѧŹŌĢā ĢāŠĶ£ŗŹµŃéĢā
£Ø14·Ö£©Ä³»ÆѧŠ”×éĄūÓĆÅØŃĪĖįŗĶ¶žŃõ»ÆĆĢŌŚ¼ÓČČĢõ¼žĻĀÖĘČ”ĀČĘų£¬²¢ĄūÓĆĀČĘų½ųŠŠÓŠ¹ŲµÄĢ½¾æŹµŃ飬¼×Ķ¬Ń§Éč¼ĘĮĖČēĶ¼ĖłŹ¾µÄŹµŃé×°ÖĆ”£
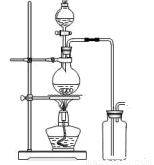
A B
£Ø1£©Š“³öÖĘČ”ĀČĘų·“Ó¦µÄĄė×Ó·½³ĢŹ½ __________________________£»×°ÖĆÖŠŹ¹ÓĆ·ÖŅŗĀ©¶·¶ų²»Ź¹ÓĆ³¤¾±Ā©¶·µÄŌŅņŹĒ_______________________________________
_____________________________________________________________________;
ŹµŃ鏱ĪŖĮĖ³żČ„ĀČĘųÖŠµÄĀČ»ÆĒāĘųĢ壬×īŗĆŌŚA”¢BÖ®¼ä°²×°Ź¢ÓŠ ŹŌ¼ĮµÄ¾»»Æ×°ÖĆ”£
£Ø2£©ČōÓĆŗ¬ÓŠ0”¢2molHClµÄMnO2·“Ó¦ÖĘĀČĘų£¬ÖʵĆCl2Ģå»ż(±ź×¼×“æöĻĀ)×ÜŹĒŠ”ÓŚ1”¢12LµÄŌŅņ ”£
£Ø3£©ŅŃÖŖ£ŗH2CO3 H++HCO3-
Ka1 =4£®45”Į10-7
H++HCO3-
Ka1 =4£®45”Į10-7
HCO3-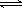 H++CO32-
Ka2=5£®61”Į10-11
H++CO32-
Ka2=5£®61”Į10-11
HClO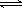 H++ClO-
Ka=2£®95”Į10-8
H++ClO-
Ka=2£®95”Į10-8
Ēėøł¾ŻŅŌÉĻĢ¼ĖįŗĶ“ĪĀČĖįµÄµēĄė³£Źż£¬Š“³öĻĀĮŠĢõ¼žĻĀ·¢Éś·“Ó¦µÄĄė×Ó·½³ĢŹ½£ŗ¢Ł½«ÉŁĮæĀČĘųĶØČė¹żĮæµÄĢ¼ĖįÄĘČÜŅŗÖŠ______________________________”£
¢ČŅŅĶ¬Ń§ČĻĪŖ¼×Ķ¬Ń§µÄŹµŃéӊȱĻŻ£¬Ģį³öŌŚ Ī»ÖĆŗó£ØĢī×ÖÄø£©Ōö¼ÓŅ»øö×°ÖĆ£¬øĆ×°ÖĆÖŠÓ¦¼ÓČė ŹŌ¼Į£¬Ęä×÷ÓĆ ”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com