
| A�����˻��ȼ��ʱ������ת����Ҫ���ɻ�ѧ��ת��Ϊ���� |
| B����ͬ�����£����������ֵ�ȱ���� |
| C����������춡�鲻�ȶ� |
| D���춡������е�̼�����������Ķ� |


 ���ʿ��ÿ��ֳɳ�ϵ�д�
���ʿ��ÿ��ֳɳ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��CH3OH(l)��3/2O2(g)===CO2(g)��2H2O(l)��H����725.8 kJ��mol��1 |
| B��2CH3OH(l)��3O2(g)===2CO2(g)��4H2O(l)��H����1452 kJ��mol��1 |
| C��2CH3OH(l)��3O2(g)===2CO2(g)��4H2O(l)��H����725.8 kJ��mol��1 |
| D��2CH3OH(l)��3O2(g)===2CO2(g)��4H2O(l)��H����1452 kJ��mol��1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����y kJ��mol��1 |
| B����(10x��y)kJ��mol��1 |
| C����(5x��0��5y)kJ��mol��1 |
| D��(10x��y)kJ��mol��1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���ɺ���ת��Ϊ���������ȷ�Ӧ��������ʱ���������Ȱ��� |
| B���ɺ���ת��Ϊ�����Ƿ��ȷ�Ӧ��������ʱ���������Ȱ��� |
| C���ɺ���ת��Ϊ�����Ƿ��ȷ�Ӧ��������ʱ���������Ȱ��� |
| D���ɺ���ת��Ϊ���������ȷ�Ӧ��������ʱ���������Ȱ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
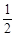 O2 (g)
O2 (g) | A���C 283.01 kJ �� mol �C 1 | B��+ 172.51 kJ �� mol �C1 |
| C��+ 283.01 kJ �� mol �C 1 | D��+ 504.00 kJ �� mol �C 1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����H3 =��H1 + 2��H2 | B����H3 =��H1 +��H2 |
| C����H3 = 2��H2����H1 | D����H3 =��H1����H2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
A��H2(g) + 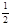 O2(g) ="=" H2O(l)����H= ��142.9kJ/mol O2(g) ="=" H2O(l)����H= ��142.9kJ/mol |
| B��2H2(g) + O2(g) ="=" 2H2O(l)����H= 571.6kJ/mol |
| C��2H2(g) + O2(g) ="=" 2H2O(l)����H= ��571.6kJ/mol |
| D��2H2 + O2 ="=" 2H2O����H= ��571.6kJ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��ij��Ӧ�ڵ������������Է����У���ô�÷�Ӧ�ڸ���������Ҳһ�����Է����� |
| B��ij��Ӧ�ڸ��������²����Է����У���ô�÷�Ӧ�ڵ���������Ҳһ�������Է����� |
| C����Ӧ���������ʱ���ر乲ͬ�����ģ��뷴Ӧ�¶��� |
| D���¶��п��ܶԷ�Ӧ�ķ�������������� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com