
��ѧʵ���ڻ�ѧѧϰ�о�����Ҫ�����á�
��1��������ʵ���йص�������ȷ����????????? (�����)��
����ˮ��Ũ������廯����������������ĥ��������ɫ����ƿ��
������ƿ����Һ©������(��)ʽ�ζ��ܵ�������ʹ��ǰ����������Ƿ�©ˮ
����ϡ���ἴ�ɼ���Na2SiO3��NaHCO3��Na[Al(OH)4]��Na2SO4������Һ
���������Ҵ��Ļ��Һ���÷�Һ©�����з���
����������ƽȷ��ȡ29��25gNaCl���壬����500mL0��5mol��L-1 NaCl��Һ
��2��ʵ������Fe2O3��CO��Ӧ����ȡ����Fe��
���밴��������������������װ��(ÿ��װ��ֻ����ʹ��һ��)��������˳��ΪA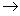 ?????? ��
?????? ��
��װ��C��������??????????? ��
��1���ڢ�? ��2����E��B��C��D? ����ȥCO�е�CO2�������ڵ�ȼCO��
��������
�����������1������ˮ�ʼ��ԣ�������������ĥ��������ɫ����ƿ������������ƿ����Һ©������(��)ʽ�ζ��ܵ�������ʹ��ǰ����������Ƿ�©ˮ����ȷ���۽�ϡ�����ֱ����Na2SiO3��NaHCO3��Na[Al(OH)4]��Na2SO4������Һ�У�����������ֱ�Ϊ������ɫ�������������塢�Ȳ�����ɫ��������ʧ���������Լ�����ȷ�����������Ҵ����ܣ������÷�Һ©�����з�����Ӧ�������룬����������ƽ�ľ�ȷ��Ϊ0.1g��������������ƽȷ��ȡ29��25gNaCl���壬����2������ʵ��Ŀ�ĺ�ʵ��ԭ�����з�������ʵ���Ŀ��Ϊ��Fe2O3��CO��Ӧ����ȡ����Fe���������װ��֪��Aװ��CO��CO2�Ļ������ͨ��NaOH��Һ��CO2�����գ����ṩ��ԭ��CO����CO�к���ˮ������Fe2O3��CO��Ӧ��Ҫ���ȣ�������ͨ��Eװ���е�Ũ�����ȥˮ������CO�����ж����������β���������õ�ȼ�ķ�����ѡDװ�ã�����������CO2�Ĵ��ڣ�Ӱ��CO�ĵ�ȼ����������Cװ���е�����������Һ��ȥβ����CO2����������˳��ΪA��E��B��C��D ����װ��C����������ȥCO�е�CO2�������ڵ�ȼCO��
���㣺���黯ѧʵ������������漰ҩƷ�ı��桢������ʹ�á����ʵļ������ʵķ��뼰ʵ��װ�õ����ӡ�


 ͬ����ϰǿ����չϵ�д�
ͬ����ϰǿ����չϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�



�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

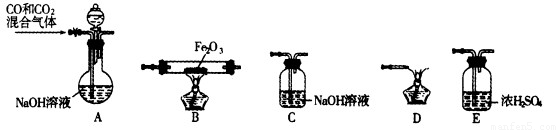
 ����Է�������Ϊ204.0���dz��õĻ����ʣ�ijѧ�����ڱ���������زⶨδ֪NaOH��Һ��Ũ�ȣ��ڱ�ʵ���дﵽ�ζ��յ�ʱ����Һ��pHԼΪ9.1��
����Է�������Ϊ204.0���dz��õĻ����ʣ�ijѧ�����ڱ���������زⶨδ֪NaOH��Һ��Ũ�ȣ��ڱ�ʵ���дﵽ�ζ��յ�ʱ����Һ��pHԼΪ9.1��| ʵ���� | NaOH��Һ�������mL�� |
| 1 | 22.52 |
| 2 | 22.49 |
| 3 | 22.50 |
| ||
(
|
| ||
(
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2006��2007ѧ��������и�����ĩ���Ի�ѧ����2007��1 ���ͣ�058
| |||||||||||||||||||||||||||||||||||||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
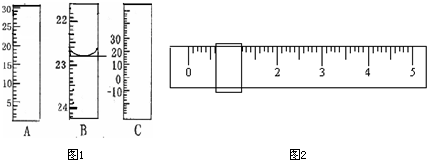

| ʵ���� | NaOH��Һ�������mL�� |
| 1 | 22.52 |
| 2 | 22.49 |
| 3 | 22.50 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com