
| Fe3+ | Fe2+ | Cu2+ | |
| �������↑ʼ����ʱ��pH | 1.9 | 7.0 | 4.7 |
| ����������ȫ����ʱ��pH | 3.2 | 9.0 | 6.7 |


 �Űٷֿ�ʱ����ϵ�д�
�Űٷֿ�ʱ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
ѡ����
����������������ѡһ������
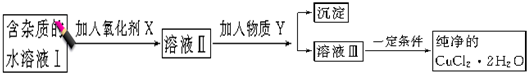
1.��������ǻ�ѧ�о����ȵ�֮һ����ѧ�����ú�����ऻ�����वĽṹʽΪ![]() �������������ڱ����Ļ��������Ϊ�м��壬ʵ����ѭ�������⣬ʾ��ͼ���£����г����ַ�Ӧ��������
�������������ڱ����Ļ��������Ϊ�м��壬ʵ����ѭ�������⣬ʾ��ͼ���£����г����ַ�Ӧ��������
��1���������ķ���ʽΪ________________________________________��
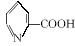
��2���������ϳɷ������£���Ӧ�����ԣ����������Ľṹδ�����⣬��Ӧʽ����ƽ����
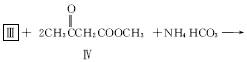
![]()
��������������_______________________��
��3���û���������ṹʽ����ͼ���������ԭ�ϣ�Ҳ�ܽ������Ƶ�������Ӧ�������л�����ĽṹʽΪ____________________��
��4������˵����ȷ����________________������ĸ����
A.��������������Ǽ��ǻ�����ȩ����2�ǻ�����ȩ
B.���������л�ԭ�ԣ�����������ԣ����ܱ����Ը��������Һ����
C.����������ɷ���ˮ�ⷴӦ
D.������������Ȼ�����ɫ�����ɷ���������Ӧ��������������ԭ��Ӧ
��5����़���������Ϊ�������ӵ���ȡ����2��़����������������Ľṹʽ����ͼ����ϳ�ԭ��2��़���ĽṹʽΪ__________________������ͬ���칹���У���ऻ���ֻ��һ����ԭ�ӱ�ȡ������़�������ͬ���칹����____________________�֡�
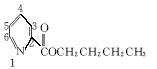
��
2.ͭ���ʼ��仯�����ںܶ���������Ҫ��;�������ͭ����������ߵ��£���ˮ����ͭ������ɱ������
��1��Cuλ��Ԫ�����ڱ��ڢ�B�塣Cu2+�ĺ�������Ų�ʽΪ_____________________��
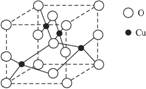
��2����ͼ��ͭ��ij��������ľ����ṹʾ��ͼ����ȷ���þ����������ӵĸ���Ϊ________��
��3������CuSO4��5H2O��д�ɣ�Cu��H2O��4��SO4��H2O����ṹʾ��ͼ���£�
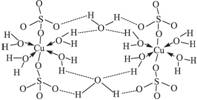
����˵����ȷ����_________________������ĸ����
A.�������ṹʾ��ͼ�У�������ԭ�Ӷ�����sp3�ӻ�
B.�������ṹʾ��ͼ�У�������λ�������ۼ������Ӽ�
C.�����Ƿ��Ӿ��壬���Ӽ�������
D.�����е�ˮ�ڲ�ͬ�¶��»�ֲ�ʧȥ
��4��������ͭ��Һ�м��������ˮ�������ɣ�Cu��NH3��4��2+�����ӡ���֪NF3��NH3�Ŀռ乹�Ͷ��������Σ���NF3������Cu2+�γ������ӣ���ԭ����_____________________��
��5��Cu2O���۵��Cu2S��______________����ߡ��͡����������ԭ��____________________________��
@@1. ��1��C11H13NO4 ��2����ȩ
��3��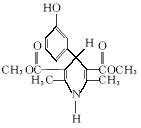
��4��BC
��5��![]() 12
12
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015�콭��ʡ������У�߶���ѧ��ѡ����ĩ��ѧ�Ծ��������棩 ���ͣ�ʵ����
��ͭ���ʼ��仯�����Ӧ�÷�Χ�ܹ㡣���к��Ȼ��������ʵ��Ȼ�ͭ����(CuCl2��2H2O),Ϊ��ȡ������CuCl2��2H2O�����Ƚ����Ƴ�ˮ��Һ��Ȼ������ͼ��������ᴿ��
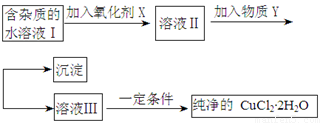
��֪Cu2+��Fe3+��Fe2+���������↑ʼ�����ͳ�����ȫʱ��pH���±���
|
|
Fe3+ |
Fe2+ |
Cu2+ |
|
�������↑ʼ����ʱ��pH |
1.9 |
7.0 |
4.7 |
|
�������������ȫʱ��pH |
3.2 |
9.0 |
6.7 |
��ش��������⣺
��1��������������Ŀ����
��2���������ʺ���������X����
A��K2Cr2O7 B��NaClO C��H2O2 D��KMnO4
��3�����������Y��
��4�������������Y��ֱ���ÿ����Լ���Һ�ܲ��ܴﵽĿ�ģ� (��ܡ����ߡ����ܡ�)�������ܣ��Խ���ԭ�� (����ܡ����˿ղ��ûش�)
��5������ܲ���ֱ�������õ�CuCl2��2H2O�� (��ܡ����ߡ����ܡ�)�������ܣ�Ӧ����β������ܵõ�CuCl2��2H2O (����ܡ����˿ղ��ûش�)
��6��������Һ���м���̼��ƣ�������������
��7��������Һ���м���þ�ۣ������������� ���Խ���ԭ��
��8��FeCl3��Һ���о�ˮ���õ�ԭ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�갲��ʡ�����ظ�����ѧ����ĩ������ѧ�Ծ��������棩 ���ͣ�ѡ����
����������Ʒ��Ӧ����㷺�Ľ������ϣ���Ԫ�صĵ��ʼ��仯������Ԫ�ػ���������ʼ�հ�������Ҫ��ɫ��
I����ĥ����ϸ��˿���ڴ����о���ȼ�գ��������ɺ�ɫ���壬�仯ѧʽΪ ��������ˮ�����ڸ�����Ҳ�����ɸú�ɫ���壬��Ӧ�Ļ�ѧ����ʽΪ ��
II��ijУ̽����ѧϰС�����Ѳ�������ķ���м����ӡˢ��·��ĸ�ʴ����������ͭ��̽���������£�
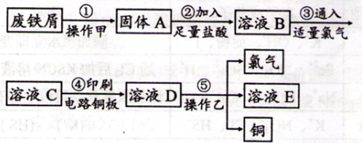
��ش��������⣺
��1��������У���������ӦFe +2HCl=FeCl2+H2���⣬�������ܷ�Ӧ�����ӷ���ʽΪ ��
��2������ҺB��ֻ����Fe2+������Fe3+����֤������ʵ��ʵ�鷽���ǣ� ��
���ȼ���ˮ�����KSCN��Һ���Ժ�ɫ��
���ȼ�KSCN��Һ�����Ժ�ɫ���ټ���ˮ���Ժ�ɫ��
�۵μ�NaOH��Һ��ֻ������ɫ������Ѹ�ٱ�Ϊ����ɫ�����ʺ��ɫ��
��ֻ�μ�KSCN��Һ���Ժ�ɫ��
A���٢� B���ڢ� C����� D���٢�
(3)����ݲ�������ͭ�Ļ�ѧ����ʽΪ ��
�����ĸ�ʴ�����µĸ������ռ�����������l/4���ࡣ�ڸ�����������ʴ�����У�������ӦʽΪ �����˹����й�������8 gFe2O3����ת�Ƶĵ��ӵ����ʵ���Ϊ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com