
CO��CO2�����Ժϳɼ״���CO��2H2��CH3OH����CO2��3H2��CH3OH��H2O
CO��CO2��H2��ͨ�����з�Ӧ�Ʊ���
A��CH4��H2O(g)��CO��3H2����B��CO��H2O(g)��CO2��H2
��ӦA�IJ���ϳɼ״�ʱH2��������ӦB�IJ���ϳɼ״�ʱH2���㣮Ϊ�˳������ԭ�Ͽɽ�������Ӧ�IJ�����ʹ�ã�
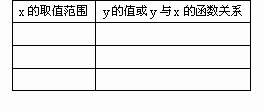
(1)��ӦA�ͷ�ӦB�IJ��������ϵ���ѱ���(��ͬ��ͬѹ����������ȱ�ʾ)
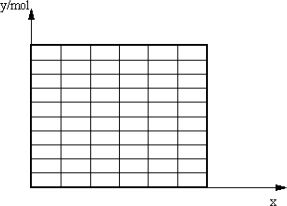
(2)��ȡA�IJ�����B�IJ���������67.2 L(��״��)������CO���������Ϊx���õ��״������ʵ���y(������)��CO��������仯�ĺ�����ϵʽ������ͼ��(�ٶ�CO����H2��Ӧ��CO��ȫ��Ӧ��CO2����H2��Ӧ)��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ���Ϻ�����У�������£�������ѧ�Ծ���3�·ݣ��������棩 ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com