
���� ��1����ȩ������������ͭ����Һ��Ӧ��������������ͭ��ˮ��
��2��������Һ��Ũ��ˮ�ķ�Ӧ����2��4��6-���屽�Ӻ��廯�⣻
��3��1-������NaOH����Һ���ȣ���Ӧ����1-�������廯�ƣ�
��4������Һ�巢��ȡ����Ӧ�����屽���廯�⣻
��5���Ҵ��Ĵ�����������ȩ��ˮ��
��� �⣺��1����ȩ������������ͭ����Һ��Ӧ��������������ͭ��ˮ����ѧ����ʽ��CH3CHO+2Cu��OH��2$\stackrel{��}{��}$Cu2O+CH3COOH+2H2O��
�ʴ�Ϊ��CH3CHO+2Cu��OH��2$\stackrel{��}{��}$Cu2O+CH3COOH+2H2O��
��2��������Һ��Ũ��ˮ�ķ�Ӧ����2��4��6-���屽�Ӻ��廯�⣬����ʽ��C6H5OH+3Br2��C6H2Br3OH��+3HBr��
�ʴ�Ϊ��C6H5OH+3Br2��C6H2Br3OH��+3HBr��
��3��1-������NaOH����Һ���ȣ���Ӧ����1-�������廯�ƣ�����ʽ��CH3 CH2 CH2Br+NaOH��CH3 CH2 CH2OH+NaBr��
�ʴ�Ϊ��CH3 CH2 CH2Br+NaOH��CH3 CH2 CH2OH+NaBr��
��4������Һ�巢��ȡ����Ӧ�����屽���廯�⣬����ʽ��C6H6+Br2$\stackrel{Fe}{��}$C6H5Br+HBr��
�ʴ�Ϊ��C6H6+Br2$\stackrel{Fe}{��}$C6H5Br+HBr��
��5���Ҵ��Ĵ�����������ȩ��ˮ������ʽ��2CH3CH2OH+O2$��_{��}^{ͭ}$2CH3CHO+2H2O��
�ʴ�Ϊ��2CH3CH2OH+O2$��_{��}^{ͭ}$2CH3CHO+2H2O��
���� ���⿼���˻�ѧ����ʽ����д�����ؿ����л���ѧ��Ӧ����ʽ��д����ȷ�л���ṹ�ص㼰�����ǽ���ؼ���ע�ⷴӦ������


 53������ϵ�д�
53������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | ���ᱵ������ˮ�������ᱵ����ǿ����� | |
| B�� | ǿ����ʶ��ǿ����Ի����������ʶ��������Ի����� | |
| C�� | �Ȼ���ˮ��Һ�ܵ��磬�����Ȼ����ǵ���� | |
| D�� | ǿ������ܹ����룬������ʲ��ܵ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Ba��OH��2��Һ�м������Al2��SO4��3��Һ��3Ba2++6OH-+2Al3++3SO42-=3BaSO4��+2Al��OH��3�� | |
| B�� | FeCl3��Һ�м�����Na2S��Һ��2Fe3++S2-=2Fe2++S�� | |
| C�� | ������ͭ��Һ�м��������ˮ��Cu2++2NH3•H2O=Cu��OH��2��+2NH4+ | |
| D�� | ��Ca��OH��2��Һ�м��������NaHCO3��Һ��Ca2++2HCO3-+2OH-=CO32-+CaCO3��+2H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| CH4 | SiH4 | NH3 | PH3 | |
| �е㣨K�� | 101.7 | 161.2 | 239.7 | 185.4 |
| �ֽ��¶ȣ�K�� | 873 | 773 | 1073 | 713.2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | OH-�ĵ���ʽ�� | |
| B�� | F-�Ľṹʾ��ͼ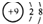 | |
| C�� | N2�Ľṹʽ�� | |
| D�� | ̼�����Ƶĵ��뷽��ʽ��NaHCO3=Na++H++CO32- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��֪2H2��g��+O2��g���T2H2O��g����H=-483.6kJ•mol-1˵��2molH2��g����1molO2��g���������ܺ�С��2molH2O��g�������� | |
| B�� | ��֪C��ʯī��s��=C�����ʯ��s������H��0������ʯ��ʯī�ȶ� | |
| C�� | ��֪NaOH��aq��+HCl��aq���TNaCl��aq��+H2O��l����H=-57.4kJ•mol-1����20gNaOH��ϡ��Һ��ϡ������ȫ�кͣ��ų�28.7kJ������ | |
| D�� | ��֪2C��s��+2O2��g���T2CO2��g����H1�� 2C��s��+O2��g��=2CO��g����H2�����H1����H2 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com