
ĻņKIČÜŅŗÖŠÖšµĪ¼ÓČėÉŁĮæCuSO4ČÜŅŗ£¬¹Ū²ģµ½ÓŠ°×É«³ĮµķÉś³É£¬ČÜŅŗ±äĪŖµ»ĘÉ«”£ŌŁĻņ·“Ó¦ŗóµÄ»ģŗĻĪļÖŠ²»¶ĻĶØČėSO2ĘųĢ壬ČÜŅŗÖš½„±ä³ÉĪŽÉ«”£ĻĀĮŠ·ÖĪöÖŠ²»ÕżČ·µÄŹĒ(””””)
A£®³ä·Ö·“Ó¦ŗóµÄČÜŅŗÖŠĪŽCu2£«“ęŌŚ
B£®µĪ¼ÓCuSO4ČÜŅŗŹ±£¬ĆæÉś³É1 mol CuI»į×ŖŅĘ1 mol e£
C£®øł¾ŻÉĻŹöŹµŃéĻÖĻóæÉÖŖ£ŗCu2£«±ČI2µÄŃõ»ÆŠŌĒæ
D£®ĶØČėSO2Ź±·¢Éś·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖSO2£«I2£«2H2O===2HI£«2H£«£«SO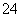
“š°ø””D
½āĪö””“ÓĻÖĻóÅŠ¶ĻæÉÖŖ·“Ó¦ŗóµÄČÜŅŗÖŠ²»“ęŌŚCu2£«(ŗ¬ÓŠCu2£«µÄČÜŅŗ³ŹĄ¶É«)£¬Ń”ĻīAÕżČ·£»øł¾Ż·“Ó¦Ź½2Cu2£«£«4I£===2CuI”ż£«I2ÖŖŃ”ĻīBÕżČ·£»øł¾ŻŃõ»Æ¼ĮµÄŃõ»ÆŠŌ±ČŃõ»Æ²śĪļµÄŃõ»ÆŠŌĒææÉÖŖCu2£«±ČI2µÄŃõ»ÆŠŌĒæ£¬Ń”ĻīCÕżČ·£»ŌŁĶØČėSO2£¬·¢Éś·“Ó¦£ŗSO2£«I2£«2H2O===2HI£«H2SO4£¬HIĪŖŅ×ČÜÓŚĖ®ĒŅŅ×µēĄėµÄĒæĖį£¬ŌŚŹéŠ“Ąė×Ó·½³ĢŹ½Ź±Ó¦ÓĆĄė×Ó·ūŗűķŹ¾£¬Ń”ĻīD“ķĪó”£



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ČēĶ¼ĖłŹ¾£¬A³ŲÓĆŹÆÄ«µē¼«µē½āpH£½13ĒāŃõ»ÆÄĘČÜŅŗ(100 mL)£¬B³Ųcµē¼«ĪŖ“æĶ£¬dµē¼«ĪŖ“ÖĶ(ŗ¬ÓŠŌÓÖŹFe”¢Ag)£¬ČÜŅŗŹĒ×ćĮæCuSO4ČÜŅŗ£¬ĶصēŅ»¶ĪŹ±¼äŗóĶ£Ö¹£¬A³Ųa¼«²śÉśµÄĘųĢåŌŚ±ź×¼×“æöĻĀĪŖ2.24 L£¬ŌņĻĀĮŠĖµ·ØÕżČ·µÄŹĒ(Ė«Ń”)(””””)”£

A£®dµē¼«ÖŹĮæŅ»¶Ø¼õÉŁ6.4 g
B£®cµē¼«ÖŹĮæŅ»¶ØŌö¼Ó6.4 g
C£®A³ŲpHŌö“ó
D£®A³ŲČÜŅŗÖŹĮæ¼õÉŁ3.6 g
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
¹¤ŅµÉĻÓĆ³ąĢśæóÉś²śĢś£¬æÉŅŌŃ”ŌńµÄ»¹Ō¼ĮÓŠCŗĶH2”£øł¾ŻĖłŃ§µÄÖŖŹ¶ÅŠ¶Ļ£ŗÄÄŅ»ÖÖ»¹Ō¼ĮæÉŹ¹·“Ó¦×Ō·¢½ųŠŠµÄĪĀ¶Č½ĻµĶ£æ
ŅŃÖŖ£ŗFe2O3(s)£«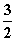 C(s)===2Fe(s)£«
C(s)===2Fe(s)£«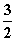 CO2(g)
CO2(g)
¦¤H£½233.8 kJ”¤mol£1£¬¦¤S£½0.279 kJ”¤mol£1”¤K£1
Fe2O3(s)£«3H2(g)===2Fe(s)£«3H2O(g)
¦¤H£½98 kJ”¤mol£1£¬¦¤S£½0.144 2 kJ”¤mol£1”¤K£1
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠÓŠ¹ŲĪļÖŹŃõ»ÆŠŌ»ņ»¹ŌŠŌĒæČõµÄŠšŹö“ķĪóµÄŹĒ(””””)
A£®Cl2”¢Br2”¢I2µÄŃõ»ÆŠŌÄÜ×÷ĪŖĀČ”¢ä唢µāŌŖĖŲ·Ē½šŹōŠŌµŻ±ä¹ęĀɵÄÅŠ¶ĻŅĄ¾Ż[2014·ø£½ØĄķ×Ū£¬23(2)b]
B£®Ķ¬Ö÷×åŌŖĖŲµÄ¼ņµ„ŅõĄė×Ó»¹ŌŠŌŌ½Ē棬Ė®½ā³Ģ¶ČŌ½“ó(2013·Ģģ½ņĄķ×Ū£¬3C)
C£®½«ÉŁĮæäåĖ®¼ÓČėKIČÜŅŗÖŠ£¬ŌŁ¼ÓČėCCl4£¬Õńµ“£¬¾²ÖĆ£¬æɹŪ²ģµ½ĻĀ²ćŅŗĢå³Ź×ĻÉ«£¬ŃéÖ¤Br2µÄŃõ»ÆŠŌĒæÓŚI2(2014·ĖÄ“ØĄķ×Ū£¬4C)
D£®½«KIŗĶFeCl3ČÜŅŗŌŚŹŌ¹ÜÖŠ»ģŗĻŗ󣬼ÓČėCCl4£¬Õńµ“£¬¾²ÖĆ£¬ĻĀ²ćČÜŅŗĻŌ×ĻŗģÉ«£¬ĖµĆ÷Ńõ»ÆŠŌ£ŗFe3£«>I2(2014·¹ć¶«Ąķ×Ū£¬22D)
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
Įņ»ÆĒā¾ßÓŠ»¹ŌŠŌ£¬æÉŅŌŗĶŠķ¶ąŃõ»Æ¼Į·“Ó¦”£ŌŚĖįŠŌĢõ¼žĻĀ£¬H2SŗĶKMnO4·“Ӧɜ³ÉS”¢MnSO4”¢K2SO4ŗĶH2O£¬Š“³öøĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
µŖŃõ»ÆŗĻĪļŹĒ“óĘųĪŪČ¾µÄÖŲŅŖŅņĖŲ£¬½«NOx×Ŗ»ÆĪŖĪŽŗ¦ĪļÖŹŹĒµ±Ē°ŃŠ¾æµÄÖŲŅŖæĪĢā”£
(1)½«NO2ŌŚŹŹµ±µÄ·“Ó¦Ģõ¼žĻĀ±ä³ÉĪŽŗ¦µÄN2£¬±ŲŠėŅŖÕŅµ½ŹŹŗĻµÄĪļÖŹG£¬GÓ¦ĪŖ________(ĢīŠ“”°Ńõ»Æ¼Į”±»ņ”°»¹Ō¼Į”±)”£
(2)ĻĀŹ½ÖŠX±ŲŠėĪŖĪŽĪŪČ¾µÄĪļÖŹ£¬ĻµŹżnæÉŅŌĪŖ0”£
NO2£«G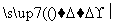 N2£«H2O£«nX(Ī“ÅäĘ½µÄ·“Ó¦Ź½)”£
N2£«H2O£«nX(Ī“ÅäĘ½µÄ·“Ó¦Ź½)”£
ĻĀĮŠ»ÆŗĻĪļÖŠ£¬Āś×ćÉĻŹö·“Ó¦Ź½ÖŠµÄGŹĒ________(ĢīŠ“×ÖÄø)”£
a£®NH3””””b£®CO2””””c£®SO2””””d£®CH3CH2OH
(3)ČōGĪŖĢģČ»ĘųµÄÖ÷ŅŖ³É·Ö£¬ŌņXĪŖ____________£¬n£½__________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŅŃÖŖ·“Ó¦¢ŁFe(s)+CO2(g)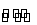 FeO(s)+CO(g)µÄĘ½ŗā³£ŹżĪŖK1£»·“Ó¦¢ŚFe(s)+H2O(g)
FeO(s)+CO(g)µÄĘ½ŗā³£ŹżĪŖK1£»·“Ó¦¢ŚFe(s)+H2O(g)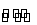 FeO(s)+H
FeO(s)+H 2(g)µÄĘ½ŗā³£ŹżĪŖK2”£ ·“Ó¦CO2(g)+H2(g)
2(g)µÄĘ½ŗā³£ŹżĪŖK2”£ ·“Ó¦CO2(g)+H2(g) 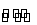 CO(g)+H2O(g)µÄĘ½ŗā³£ŹżKĪŖ( )
CO(g)+H2O(g)µÄĘ½ŗā³£ŹżKĪŖ( )
A.£Ė1+£Ė2 B.K1-K2 C.£Ė1”¤ £Ė2 D.£Ė1/£Ė2
£Ė2 D.£Ė1/£Ė2
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŅŃÖŖ£ŗŗ¬C£½CµÄĪļÖŹ( Ļ©Ģž)Ņ»¶ØĢõ¼žĻĀÄÜÓėĖ®·¢Éś¼Ó³É·“Ó¦£¬Éś³É“¼(ŗ¬ōĒ»łµÄĢžµÄŃÜÉśĪļ)£»ÓŠ»śĪļA”«D¼ä“ęŌŚĶ¼Ź¾µÄ×Ŗ»Æ¹ŲĻµ”£ĻĀĮŠĖµ·Ø²»ÕżČ·µÄŹĒ
( )

A.ĪļÖŹD½į¹¹¼ņŹ½ĪŖCH3COOCH2CH3
B.ĪļÖŹCÄÜŹ¹ĖįŠŌøßĆĢĖį¼ŲČÜŅŗĶŹÉ«
C.BÓėŅŅĖį·¢ÉśĮĖČ”“ś·“Ó¦
D.æÉÓĆBŻĶČ”µāĖ®ÖŠµÄµāµ„ÖŹ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ij»ģŗĻĪļA,ŗ¬ÓŠKAl(SO4)2”¢Al2O3ŗĶFe2O3,ŌŚŅ»¶ØĢõ¼žĻĀæÉŹµĻÖČēĶ¼ĖłŹ¾µÄĪļÖŹÖ®¼äµÄ±ä»Æ:
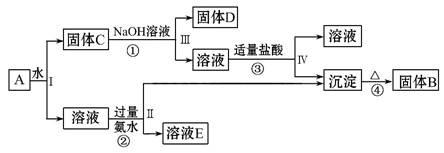
¾Ż“Ė»Ų“šĻĀĮŠĪŹĢā:
(1)¢ń”¢¢ņ”¢¢ó”¢¢ōĖIJ½ÖŠ¶ŌÓŚČÜŅŗŗĶ³ĮµķµÄ·ÖĄė²ÉČ”µÄ·½·ØŹĒ __________”£
(2)øł¾ŻÉĻŹöæņĶ¼·“Ó¦¹ŲĻµ,Š“³öĻĀĮŠB”¢C”¢D”¢EĖłŗ¬ĪļÖŹµÄ»ÆѧŹ½:
¹ĢĢåB____________________________________________________________;
¹ĢĢåC____________________________________________________________;
¹ĢĢåD____________________________________________________________;
ČÜŅŗE____________________________________________________________”£
(3)¢Ł¢Ū·“Ó¦µÄ»Æѧ·½³ĢŹ½·Ö±šĪŖ: ___________________________________”¢
__ __________________________________ӣ
¢Ś·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ _____________________________________________”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com