
Ϊ���о���������Թ�������ֽ����ʵ�Ӱ�죬ijͬѧ��������ʵ�飬��ش��������⡣
|
��� |
���� |
ʵ������ |
|
�� |
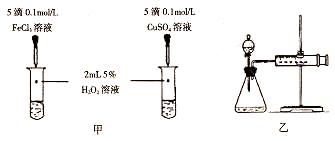
�ֱ����Թ�A��B�м���5 mL 5% H2O2��Һ��������2��1mol/L FeCl3��Һ�����Թ��о����������ݳ���ʱ�����Թ�A����ʢ��5��������ˮ���ձ��н��ݣ����Թ�B����ʢ��40��������ˮ���ձ��н��ݡ� |
�Թ�A�в��ٲ������ݣ� �Թ�B�в��������������� |
|
�� |
��ȡ��֧�Թֱܷ����5 mL 5% H2O2��Һ��5 mL 10% H2O2��Һ |
�Թ�A��B�о�δ���Լ��������ݲ����� |
��1����������ֽ�Ļ�ѧ����ʽΪ_____________________________________��
��2��ʵ��ٵ�Ŀ����_________________________________________________��
ʵ���еμ�FeCl3��Һ��Ŀ����_______________________________________��
��3��ʵ���δ�۲쵽Ԥ�ڵ�ʵ������Ϊ�˰�����ͬѧ�ﵽʵ��Ŀ�ģ�������Ķ����������ĸĽ������_______________________����ʵ�������ṩ�ļ����Լ�����
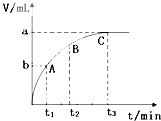
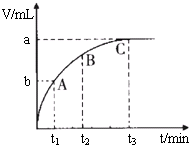
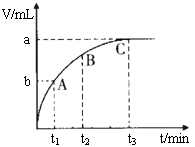
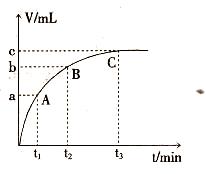
��4��ijͬѧ��50 mLһ��Ũ�ȵ�H2O2��Һ�м���һ�����Ķ������̣��ų�������������״���£��뷴Ӧʱ��Ĺ�ϵ����ͼ��ʾ����A��B��C��������ʾ��˲ʱ��Ӧ������������______��

��1��2H2O2 2H2O+O2��
2H2O+O2��
��2���о��¶ȶ�H2O2�ֽ����ʵ�Ӱ�� �ӿ�H2O2�ֽ����ʣ�ʹʵ���������ڹ۲�
��3������֧�Թ�ͬʱ����ʢ����ͬ�¶���ˮ���ձ��У�������֧�Թ���ͬʱ����2��1mol/LFeCl3��Һ���۲�������ݵ�����
��4��C��
��������
�����������1���ڴ����������£���������ֽ�����������ˮ����Ӧ�Ļ�ѧ����ʽ��2H2O2 2H2O+O2����
2H2O+O2����
��2���ֱ����Թ�A��B�м��� 5mL 5% H2O2��Һ��������1��2 ��1mol/L FeCl3��Һ�����Թ��о����������ݳ��֣�˵����������ֽ��ܷ������Թ�A��B�о����������ݳ���ʱ�����Թ�A����ʢ��5��������ˮ���ձ��У����Թ�B����ʢ��40��������ˮ���ձ��У���֧�Թܲ�ͬ�����Թ�A���¶ȱ��Թ�B���¶ȵͣ�˵���о������¶ȶԷ�Ӧ���ʵ�Ӱ�죬����ʼ�ӵμ�FeCl3��Һ��Ŀ�ļӿ�H2O2�ֽ⡣���ʵ��ٵ�Ŀ�����о��¶ȶ�H2O2�ֽ����ʵ�Ӱ�죻�������Ȼ����������Ǽӿ�H2O2�ֽ����ʣ�ʹʵ���������ڹ۲졣
��3��Ӱ�컯ѧ��Ӧ���ʵ����������Ũ�ȡ��¶ȡ������ѹǿ������������ı�����ȡ����Ϊ�ӿ췴Ӧ���ʣ��ɴ��¶ȡ��������Ӱ��Ƕȿ��ǣ�������֧�Թ�ͬʱ����ʢ����ͬ�¶���ˮ���ձ��У�������֧�Թ���ͬʱ����2��1mol/L FeCl3��Һ���۲�������ݵ����ʣ�
��4����ͼ��������ʾʱ�䣬�������ʾ��������������ʱ��Խ�����ɵ�����Խ�࣬��Ӧ����Խ�죬���ߵ�б��Խ����������������ΪC��
���㣺������������Է�Ӧ����Ӱ���ʵ��̽��
�����������Ǹ߿��еij������ͣ����ڻ���������Ŀ��飬�ѶȲ����ʱ��ע����ȷʵ���ԭ��������Ӱ�췴Ӧ���ʵ����������һ���з������ɣ�����������ѧ������˼ά�����淶�Ͻ���ʵ�����������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 Ϊ���о���������Թ�������ֽ����ʵ�Ӱ�죬ijͬѧ��������ʵ�飬��ش��������⣮
Ϊ���о���������Թ�������ֽ����ʵ�Ӱ�죬ijͬѧ��������ʵ�飬��ش��������⣮| ��� | �� �� | ʵ������ |
| �� | �ֱ����Թ�A��B�м��� 5mL 5% H2O2��Һ��������1��2 ��1mol/L FeCl3��Һ�����Թ��о����������ݳ���ʱ�����Թ�A����ʢ��5��������ˮ���ձ��У����Թ�B����ʢ��40��������ˮ���ձ��У� | �Թ�A�в��ٲ������ݣ��Թ�B�в��������������� |
| �� | ��ȡ��֧�Թֱܷ���� 5mL 5%H2O2��Һ�� 5mL 10%H2O2��Һ�� |
�Թ�A��B�о�δ�����ݲ��� |
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ��� | ���� | ʵ������ |
| �� | �ֱ����Թ�A��B�м���5mL 5% H2O2��Һ��������2��1mol/L FeCl3��Һ�����Թ��о����������ݳ���ʱ�����Թ�A����ʢ��5��������ˮ���ձ��н��ݣ����Թ�B����ʢ��40��������ˮ���ձ��н��ݣ� | �Թ�A�в��ٲ������ݣ� �Թ�B�в��������������� |
| �� | ��ȡ��֧�Թֱܷ����5mL 5% H2O2��Һ��5mL 10% H2O2��Һ | �Թ�A��B�о�δ���Լ��������ݲ����� |
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��㶫ʡտ���и�һ��ѧ����ĩ���п��Ի�ѧ�Ծ����������� ���ͣ������
Ϊ���о���������Թ�������ֽ����ʵ�Ӱ�죬ijͬѧ��������ʵ�飬��ش��������⡣
| ��� | ���� | ʵ������ |
| �� | �ֱ����Թ�A��B�м���5 mL 5% H2O2��Һ��������2��1 mol/L FeCl3��Һ�����Թ��о����������ݳ���ʱ�����Թ�A����ʢ��5��������ˮ���ձ��н��ݣ����Թ�B����ʢ��40��������ˮ���ձ��н��ݡ� | �Թ�A�в��ٲ������ݣ� �Թ�B�в��������������� |
| �� | ��ȡ��֧�Թֱܷ����5 mL 5% H2O2��Һ��5 mL 10% H2O2��Һ | �Թ�A��B�о�δ���Լ��������ݲ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��㶫ʡʵ����ѧ��һ��ѧ����ĩ���Ի�ѧ�Ծ����������� ���ͣ�ʵ����
��10�֣�Ϊ���о���������Թ�������ֽ����ʵ�Ӱ�죬ijͬѧ��������ʵ�飬��ش��������⡣
| ��� | ���� | ʵ������ |
| �� | �ֱ����Թ�A��B�м���5 mL 5% H2O2��Һ��������2��1 mol/L FeCl3��Һ�����Թ��о����������ݳ���ʱ�����Թ�A����ʢ��5��������ˮ���ձ��н��ݣ����Թ�B����ʢ��40��������ˮ���ձ��н��ݡ� | �Թ�A�в��ٲ������ݣ� �Թ�B�в��������������� |
| �� | ��ȡ��֧�Թֱܷ����5 mL 5% H2O2��Һ��5 mL 10% H2O2��Һ | �Թ�A��B�о�δ���Լ��������ݲ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�꽭��ʡ�����и�һ��ѧ����ĩ��ѧ�������ۻ�ѧ�Ծ����������� ���ͣ�ʵ����
Ϊ���о����������H2O2�ֽ����ʵ�Ӱ�죬ijͬѧ��������ʵ�飬��ش��������⡣
| ��� | ���� | ʵ������ |
| �� | �ֱ����Թ�A��B�м���5mL 5%H2O2��Һ��������2��1 mol/L FeCl3��Һ�����Թ��о����������ݳ���ʱ�����Թ�A����ʢ��5��������ˮ���ձ��У����Թ�B����ʢ��40��������ˮ���ձ��С� | �Թ�A�в������������٣��Թ�B�в��������������� |
| �� | ��ȡ��֧�Թֱܷ����5mL 5%H2O2��Һ��5mL 10%H2O2��Һ | �Թ�A��B�о�δ���Լ��������ݲ����� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com