
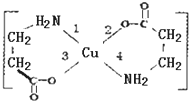
 II£®¶žøŹ°±ĖįŗĻĶ£ØII£©ŹĒ×īŌē±»·¢ĻֵĵēÖŠŠŌÄŚÅäŃĪ£¬ĖüµÄ½į¹¹ČēĶ¼£ŗ
II£®¶žøŹ°±ĖįŗĻĶ£ØII£©ŹĒ×īŌē±»·¢ĻֵĵēÖŠŠŌÄŚÅäŃĪ£¬ĖüµÄ½į¹¹ČēĶ¼£ŗ·ÖĪö £Ø3£©ĶŹĒ29ŗÅŌŖĖŲ£¬øł¾ŻŗĖĶāµē×ÓÅŲ¼¹ęĀɏ銓»łĢ¬Cu2+µÄ×īĶā²ćµē×ÓÅŲ¼Ź½£»
£Ø4£©øł¾ŻµŚŅ»µēĄėÄÜŗĶŌŖĖŲµÄ·Ē½šŹōŠŌÅŠ¶Ļ£»
£Ø5£©Ė«¼üÖŠŗ¬ÓŠ1øö¦Š¼ü£¬øł¾ŻĖ«¼üµÄŹżÄæÅŠ¶Ļ£»
£Ø6£©øł¾ŻŌ×ӵijɼüĢŲµć£¬¼°ÅäĪ»¼üµÄŠĪ³ÉĢõ¼ž·ÖĪö£»
½ā“š ½ā£ŗ£Ø3£©CuŹĒ29ŗÅŌŖĖŲ£¬Ō×ÓŗĖĶāµē×ÓŹżĪŖ29£¬»łĢ¬Ō×ÓŗĖĶāµē×ÓÅŲ¼Ź½ĪŖ£ŗ1s22s22p63s23p63d104s1£¬ĶŌ×ÓŹ§Č„4s¼°3dÉĻø÷Ņ»øöµē×ÓŠĪ³ÉCu2+£¬¹ŹCu2+Ąė×ӵĵē×ÓÅŲ¼Ź½ĪŖ£ŗ1s22s22p63s23p63d9£¬»łĢ¬Cu2+µÄ×īĶā²ćµē×ÓÅŲ¼Ź½ĪŖ3d9£¬
¹Ź“š°øĪŖ£ŗ3d9£»
£Ø4£©ŅŃÖŖ¶žøŹ°±ĖįŗĻĶ£Ø¢ņ£©µÄ½į¹¹Ķ¼£¬ĘäÖŠµŚŅ»µēĄėÄÜ×ī“óµÄĪŖNŌŖĖŲ£¬ŌŖĖŲµÄ·Ē½šŹō×īŠ”µÄ·Ē½šŹōĪŖHŌŖĖŲ£¬¶žÕߊĪ³ÉµÄ5ŗĖ10µē×ÓµÄĪ¢Į£ĪŖNH4+£¬øĆĪ¢Į£µÄæռ乹ŠĶŹĒÕżĖÄĆęĢ壬
¹Ź“š°øĪŖ£ŗÕżĖÄĆęĢ壻
£Ø5£©ŅŃÖŖĖ«¼üÖŠŗ¬ÓŠ1øö¦Š¼ü£¬ÓɶžøŹ°±ĖįŗĻĶ£Ø¢ņ£©µÄ½į¹¹Ķ¼æÉÖŖ£¬1mol¶žøŹ°±ĖįŗĻĶ£Ø¢ņ£©ŗ¬ÓŠ2molC=O£¬Ōņŗ¬ÓŠµÄ¦Š¼üŹżÄæŹĒ2NA»ņ1.204”Į1024£¬
¹Ź“š°øĪŖ£ŗ2NA»ņ1.204”Į1024£»
£Ø6£©CuŌ×Óŗ¬ÓŠæÕ¹ģµĄÄÜÓėĘäĖüŌ×ÓŠĪ³ÉÅäĪ»¼ü£¬ÓÉÓŚOŌ×ÓŌŚ»ÆŗĻĪļÖŠÄÜŠĪ³É2øö¹²¼Ū¼ü£¬ĖłŅŌOÓėCuŠĪ³É¹²¼Ū¼ü£¬NÓėCuŠĪ³ÉÅäĪ»¼ü£¬¼“ŹōÓŚÅäĪ»¼üµÄŹĒ1ŗĶ4£¬
¹Ź“š°øĪŖ£ŗ1ŗĶ4£»
µćĘĄ ±¾Ģāæ¼²éĮĖµē×ÓÅŲ¼Ź½µÄŹéŠ“”¢µŚŅ»µēĄėÄÜ”¢Ī¢Į£µÄæÕ¼ä½į¹¹”¢ÅäĪ»¼üµČÖŖŹ¶£¬ĢāÄæÄѶČÖŠµČ£¬×¢Ņā¶ŌĢāÖŠĖłøų½į¹¹Ķ¼µÄ·ÖĪöŹĒ½āĢāµÄ¹Ų¼ü£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | CH3COOH+CH3CH2OH$”ś_{”÷}^{ÅØH_{2}SO_{4}}$CH3COOCH2CH3+H2O | |
| B£® | 2CH3CH2OH+O2$”ś_{”÷}^{“߻ƼĮ}$2CH3CHO+2H2O | |
| C£® | CH4+Cl2$\stackrel{¹āÕÕ}{”ś}$CH3Cl+HCl | |
| D£® |  +Br2$\stackrel{FeBr_{3}}{”ś}$ +Br2$\stackrel{FeBr_{3}}{”ś}$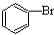 +HBr +HBr |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
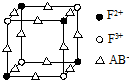 ŅŃÖŖA”¢B”¢C”¢D”¢E”¢F¶¼ŹĒÖÜĘŚ±ķÖŠĒ° ĖÄÖÜĘŚµÄŌŖĖŲ£¬ĖüĆĒµÄŗĖµēŗÉŹżŅĄ“ĪŌö“ó£¬ĘäÖŠA”¢B”¢C”¢D”¢EĪŖ²»Ķ¬Ö÷×åµÄŌŖĖŲ£®A”¢CµÄ×īĶā²ćµē×ÓŹż¶¼ŹĒĘäµē×Ó²ćŹżµÄ2±¶£¬BµÄµēøŗŠŌ“óÓŚC£¬Ķø¹żĄ¶É«īܲ£Į§¹Ū²ģEµÄŃęÉ«·“Ó¦ĪŖ×ĻÉ«£¬FµÄ»łĢ¬Ō×ÓÖŠÓŠ4øöĪ“³É¶Ōµē×Ó£®
ŅŃÖŖA”¢B”¢C”¢D”¢E”¢F¶¼ŹĒÖÜĘŚ±ķÖŠĒ° ĖÄÖÜĘŚµÄŌŖĖŲ£¬ĖüĆĒµÄŗĖµēŗÉŹżŅĄ“ĪŌö“ó£¬ĘäÖŠA”¢B”¢C”¢D”¢EĪŖ²»Ķ¬Ö÷×åµÄŌŖĖŲ£®A”¢CµÄ×īĶā²ćµē×ÓŹż¶¼ŹĒĘäµē×Ó²ćŹżµÄ2±¶£¬BµÄµēøŗŠŌ“óÓŚC£¬Ķø¹żĄ¶É«īܲ£Į§¹Ū²ģEµÄŃęÉ«·“Ó¦ĪŖ×ĻÉ«£¬FµÄ»łĢ¬Ō×ÓÖŠÓŠ4øöĪ“³É¶Ōµē×Ó£® ]-£¬ĘäÖŠŠÄŌ×Ó²ÉÓĆspŌӻƣ®
]-£¬ĘäÖŠŠÄŌ×Ó²ÉÓĆspŌӻƣ®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | 1 molNa2O2ÖŠŗ¬ÓŠµÄĄė×Ó×ÜŹżĪŖ4 NA | |
| B£® | 0.1 molōĒ»ł£Ø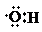 £©ÖŠŗ¬ÓŠµÄµē×ÓŹżĪŖ0.7 NA £©ÖŠŗ¬ÓŠµÄµē×ÓŹżĪŖ0.7 NA | |
| C£® | ŹŅĪĀĻĀ£¬1 L pH=13µÄNaOHČÜŅŗÖŠ£¬Ė®µēĄė³öµÄOH-µÄŹżÄæĪŖ0.1 NA | |
| D£® | ŌŚ5NH4NO3$\frac{\underline{\;\;”÷\;\;}}{\;}$2HNO3+4N2”ü+9H2OÖŠ£¬Éś³É28 g N2Ź±×ŖŅʵĵē×ÓŹżÄæĪŖ3.75 NA |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
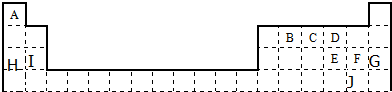
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Ć÷·ÆæÉÓĆÓŚ×ŌĄ“Ė®µÄĻū¶¾¾»»Æ | |
| B£® | Fe2 O3æÉÓĆ×÷ŗģÉ«ÓĶĘįŗĶĶæĮĻ | |
| C£® | ·ÓČ©Ź÷Ö¬æÉÓĆ×÷¾ųŌµ”¢øōČČŗĶø“ŗĻ²ÄĮĻ | |
| D£® | ĮņĖįæÉÓĆÓŚ¾«Į¶ŹÆÓĶŅŌ¼°ÖĘČ”¶ąÖÖ»Ó·¢ŠŌĖį |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | 35.5£ŗ108 | B£® | 1£ŗ8 | C£® | 108£ŗ35.5 | D£® | 137£ŗ71 |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com