
����������Ҫ�ľ�ϸ����ԭ��,��ҽҩ��Ⱦ�ϵ��м��壬�������л��ܼ����Ʊ��������Ĺ������£������ƻ����װ����ͼ��Ӧװ�á�
ȡ100 mL�ձ�����20 mLŨ������Ũ����18 mL���ƻ���ᣬ����©���С���18 mL������������ƿ�С�
���������µı�����μ�����ᣬ�ߵα߽��裬��;��ȡ�
����50-60���·�����Ӧ��ֱ����Ӧ������
�ܳ�ȥ�����ֲ�Ʒ����������ˮ��10%Na2CO3��Һϴ�ӣ������������ˮϴ�ӵõ��ֲ�Ʒ��
��֪��1��

��2�������õ����й������б�����
| ���� | �۵�/�� | �е�/�� | �ܶ�(20 ��) / g��cm-3 | �ܽ��� |
| �� | 5.5 | 80 | 0.88 | ����ˮ |
| ������ | 5.7 | 210.9 | 1.205 | ������ˮ |
| 1,3-�������� | 89 | 301 | 1.57 | ����ˮ |
| Ũ���� |  | 83 | 1.4 | ������ˮ |
| Ũ���� |  | 338 | 1.84 | ������ˮ |
��1��Ũ����
��2�����Ա���©����ѹǿ�뷢������ѹǿ��ȣ�ʹ©����Һ����˳������
��3�������ܣ����������ܻ�ֱ�������ܾ��ɣ�
��4���� ��Һ
��5��ȡ���һ��ϴ��Һ������Һ�м����Ȼ��ƣ��������ɣ�˵����ϴ����
��6�� �Ȼ���
���������������1������Ũ������ܶȱ�Ũ����Ĵ�Ũ������ˮʱ�ų��������ȣ��������û���Ӧ��Ũ������뵽Ũ�����У�Ҳ���������ձ����ȼ���Ũ���ᡣ��2�������ڷ�Ӧ�Ĺ����в��ϼ��ȣ���������������壬�����Һ��ļ���ܲ����������ú�ѹ��Һ©���μӣ����Ա���©����ѹǿ�뷢������ѹǿ��ȣ�ʹ©����Һ����˳�����¡���3����ʵ��Ĺ����б����������Ϊ���ȶ��������������ʵ��˷��뻷����Ⱦ��������װ���г������ܿ���������������ʹ���ʻ��������á���˿��������ܵ�����װ�ô��档��4����Ӧ�������������������1,3-�������������ܽ���ˮ��Һ�壬�ܶȱ���Ļ����ҺС�����Է�Ӧ�������Ʒ��Һ����ϲ㣬���뻥�����ܵ�����Һ��ķ����Ƿ�Һ����5�����ڵõ��Ĵֲ�Ʒ����������ˮ��10%Na2CO3��Һϴ�ӣ������������ˮϴ�ӡ����ϴ�Ӹɾ�����ϴ��Һ�в�����CO32-�����Լ���Һ����ϴ���ķ�����ȡ���һ��ϴ��Һ������Һ�м����Ȼ��ƣ����������ɣ�˵����ϴ������6��Ϊ�˵õ�����������������ʹ֮������ˮ����������Һ���м�������ˮ�������õ���ˮCaCl2����ȥˮ��Ȼ�����͵õ��˲�Ʒ��
���㣺������Ҫ�ľ�ϸ����ԭ������������ȡ�������漰�����ʵĻ�ϡ������ķ��롢���ʵ�ϴ�ӡ������ʵ�������֪ʶ��


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
������ʾ��þ�뱥��̼��������Һ��Ӧ������������Ͱ�ɫ�����ijͬѧ���������ʵ�鷽��̽����Ӧԭ������֤���
(1)�������
ʵ�����ɰֽ��ȥþ����������Ĥ���������ʢ���������з�̪�ı���̼��������Һ���Թ��У�Ѹ�ٷ�Ӧ�������������ݺͰ�ɫ�������Һ��dz���졣
��ͬѧ�Է�Ӧ�в����İ�ɫ�������������²²⣺
�²�1����ɫ���������Ϊ________��
�²�2����ɫ���������ΪMgCO3��
�²�3����ɫ����������Ǽ�ʽ̼��þ[xMgCO3��yMg(OH)2]��
(2)��ƶ���ʵ��ȷ�����ﲢ��֤�²⣺
| ʵ����� | ʵ�� | ʵ������ | ���� |
| ʵ��� | ��ʵ������� �����������ȼ | �ܰ���ȼ�ա��� ������ɫ���� | ����ɷ�Ϊ __��__ |
| ʵ��� | ȡʵ����еİ� ɫ�����ϴ�ӣ� ��������__��__ | __��__ | ��ɫ��������ܺ���MgCO3 |
| ʵ��� | ȡʵ����еij� ��Һ�������м��� ����CaCl2ϡ��Һ | ������ɫ���� | ��Һ�д���__��__ |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��˾ƥ�ֿ���ˮ�����������������Ƶá����Ʊ�ԭ�����£�

��˾ƥ��(����ˮ����)������������ˮ����˾ƥ�ֿɰ����²�����ȡ�ʹ�����
����1���ڸ����50 mLԲ����ƿ�м���2 gˮ���ᡢ5 mL��������5��Ũ���ᣬ��ʹˮ����ȫ���ܽ⡣
����2����ͼ��ʾװ��װ���������ͨˮ����ˮԡ�ϼ��Ȼ���5��10 min������ˮԡ�¶���85��90 �档
����3����Ӧ������ȡ�·�Ӧƿ����ȴ���ٷ����ˮ����ȴ���ᾧ�����ˡ���ˮϴ��2��3�Σ��������˵ôֲ��
����4�����ֲ���ת����150 mL�ձ��У��ڽ����¼���25 mL����̼��������Һ����ֽ��裬Ȼ����ˡ�
����5������Һ����10 mL 4 mol��L��1���ᣬ���裬���ձ����ڱ�ԡ����ȴ��ʹ�ᾧ��ȫ�����ˣ�������ˮϴ��2��3�Ρ�
(1)����1Ũ��������ÿ�����________��
(2)����2�У�������ͨˮ��ˮӦ��________�ڽ�(�a����b��)��
(3)����3����ʱ����ʱ��ֽ�ᴩ�ף�������ֽ���Ĵ�ʩ��______________________________________________________________��
(4)����4������Ҫ��Ӧ�Ļ�ѧ����ʽΪ_____________________�����˵õ��Ĺ���Ϊ________��
(5)ȡ��������5��õľ������ʢ��5 mLˮ���Թ��У�����1��2��1%���Ȼ�����Һ��������Һ����ɫ���ɲ���________��������һ���������塣
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�������Ʊ���Ϊ��ҵ�Σ���Ư�ס���Ƶȷ���Ӧ�ù㷺����ľ̿��Ũ���ᡢˮ��ͭΪԭ�����ɵ�һ��������������Ʒ�Ӧ�Ʊ��������Ƶ�װ������ͼ��ʾ�����ּг�װ���ԣ���
��֪�������£���2NO+Na2O2��2NaNO2 ��3NaNO2+3HCl��3NaCl+HNO3+2NO��+H2O��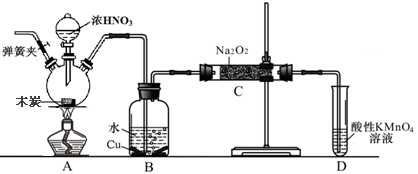
�����������£�NO��NO2�C������MnO4�C��Ӧ����NO3�C��Mn2+
���������գ�
��1��д��Ũ������ľ̿��Ӧ�Ļ�ѧ����ʽ ��
��2��B�й۲쵽����Ҫ������ ��Dװ�õ������� ��
��3������C�в������������Ƶķ����� ��
��4��������C�����г�������������и�����̼���ƺ� ��Ϊ���������Щ������Ӧ��B��Cװ�ü�����װ��E������E��ע��E��ʢ�ŵ�ҩƷ���� ��
��5��д������C�������Ƿ�̼���Ƶķ��� ��
��6����1.56g����������ȫת����Ϊ�������ƣ�������������Ҫľ̿ g��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�������ƣ�NaClO2����һ�ָ�Ч��������Ư������֪��NaClO2������Һ���¶ȵ���38��ʱ�����ľ�����NaClO2��3H2O������38��ʱ����������NaClO2������60��ʱNaClO2�ֽ��NaClO3��NaCl��������ͼ��ʾװ���Ʊ��������ơ�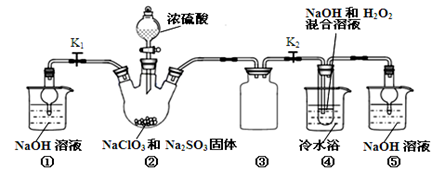
���������գ�
��1��װ�â��в���ClO2�Ļ�ѧ����ʽΪ ��װ�â۵������� ��
��2����װ�âܷ�Ӧ�����Һ���NaClO2����IJ�������Ϊ��
�ټ�ѹ��55�������ᾧ���� ���� ���ܵ���60�����õ���Ʒ��
��3��ȷ��ȡ��������������Ʒ10g���ձ��У�������������ˮ�����ĵ⻯�ؾ��壬�ٵ���������ϡ���ᣬ��ַ�Ӧ��ClO2��+ 4I��+4H+ ��2H2O+2I2+Cl�����������û��Һ���250mL������Һ�����ƴ���Һ���õ��Ķ������������� ��
��4��ȡ25.00mL����Һ����2.0 mol/L Na2S2O3��Һ�ζ���I2 +2S2O32����2I��+S4O62�������Ե�����Һ��ָʾ�����ﵽ�ζ��յ�ʱ������Ϊ ���ظ��ζ�2�Σ����Na2S2O3��Һƽ��ֵΪ20.00 mL������Ʒ��NaClO2����������Ϊ ��
��5��ͨ������˵��װ�â��ڱ�ʵ���е����� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ijС�������ͼװ�ã����ּг�װ������ȥ������̽����ʪ��Cl2��Na2CO3��Ӧ�õ��������ʵijɷ֡�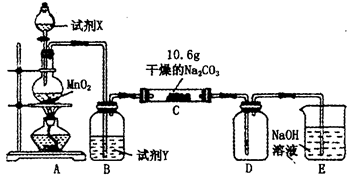
��1��A����ȡCl2�����ӷ���ʽ_______________________________________.
��2��ͨ��һ������ʪ��Cl2��Ӧ����⣬D��ֻ��Cl2Oһ�����壬C��ֻ��һ�������⣬ͬʱ����NaHCO3�ȣ�ijͬѧ��C�����ù�������ijɷֽ���̽����
������������衣
����1���������ֳɷ֣�NaHCO3��_____________________________��
����2���������ֳɷ֣�NaHCO3��_____________________________��
����Ʒ���������ʵ�顣д��ʵ�鲽���Լ�Ԥ������ͽ��ۡ�
��ѡʵ���Լ�������������ˮ��ϡHNO3��BaCl2��Һ������ʯ��ˮ��AgNO3��Һ���Թܡ�С�ձ���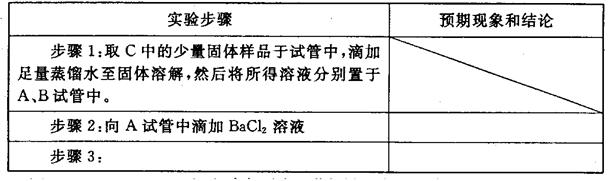
��3����֪C����0��1 mol Cl2�μӷ�Ӧ��������һ����������֪C�з�Ӧ�Ļ�ѧ����ʽΪ__________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�о���ѧϰС���ij�����������壨FeSO4?xH2O���ȷֽ��о�����С��ͬѧ��ȡag��������������Ʒ��ͼ1���¼��ȣ�ʹ����ȫ�ֽ⣬�����ò������̽������ͨ������װ��B�������x��ֵ��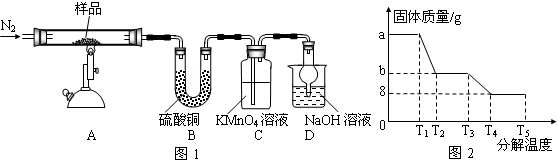
��1��װ��B������ͭ��ĩ��������������12.6g��˵����������ˮ��װ��C�и��������Һ��ɫ��˵�������л��� ��
��2��ʵ����Ҫ����ͨ�뵪������������x�� ���ƫ����ƫС�����䡱����
��3����������������ȫ�ֽ��װ��A�л���������ɫ����Fe2O3��
��4���������Ϸ����ó����������ֽ������һ����SO3��д��FeSO4�ֽ�Ļ�ѧ����ʽ ��
��5��װ��D���θ���ܵ����� ��
��6��ij�о�������SDTQ600�ȷ����Ƕ������������壨FeSO4?xH2O�������ȷֽ⣬���������ݣ����Ƴɹ������������ֽ��¶ȵĹ�ϵͼ��ͼ2������ͼ2���й����ݣ��ɼ����FeSO4?xH2O�е�x= ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��ij��ѧ�С���������ͼ��ʾ�����ּг�װ������ȥ��ʵ��װ�ã���̽����ʪ��Cl2�����Na2CO3 ���巴Ӧ�õ��Ĺ������ʵijɷ֡�
��֪��ͨ��һ�������������D��ֻ��һ�ֳ�����Ϊ�ƺ�ɫ�����壬��Ϊ�������������ȷ������C�й��庬��NaHCO3 ���Һ��ȵ���ֻ��һ�֡��ֶ�C�ijɷֽ��в����̽����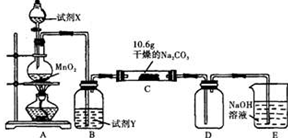
��1������������룺����֪C����0.1molCl2ǡ�ú�10.6��Na2CO3������ȫ��Ӧ����C����Cl2���뷴Ӧ�Ļ�ѧ����ʽ���� ��
��2��������������衣
����1���������ֳɷ֣�NaHCO3�� ��
����2���������ֳɷ֣�NaHCO3�� �� ��
����ƺ���������C�����е�δ֪�ɷֽ���̽������д��ʵ�鲽���Լ�Ԥ������ͽ��ۣ��ɲ���������
��ѡʵ���Լ�������������ˮ��ϡHNO3��Ba(OH)2��Һ��BaCl2��Һ������ʯ��ˮ��AgNO3��Һ���Թܡ�С�ձ���
| ʵ�鲽�� | Ԥ������ͽ��� |
| ����1��ȡ����C�й�����Ʒ���Թ��У�������������ˮ���������������ȫ�ܽ⣬Ȼ��������Һ��װA��B��֧�Թ��С� | |
| ����2�� | |
| ����3�� | |
| | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ʵ�����Ʊ���������泥�NH2COONH4���ķ�Ӧ���£�2NH3(g)��CO2(g)  NH2COONH4(s)���÷�Ӧ�ڸ��������½����ɰ�������泥�����ˮ����������̼��炙�̼����李�
NH2COONH4(s)���÷�Ӧ�ڸ��������½����ɰ�������泥�����ˮ����������̼��炙�̼����李�
��1���÷�Ӧ��һ���������ܹ��Է����У���Ӧ�Ħ�H 0��������ڡ�С�ڻ���ڣ�
��2��д������̼����淋Ļ�ѧ����ʽ ��
��3������ͼװ�ý�������ʵ�飺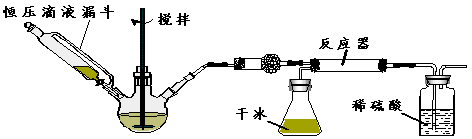
����1�����װ�������ԡ�
����2������Ӧ������װ��ҩƷ��������������ƿ�м����������������ƹ��壬��ѹ��Һ©����װ��Ũ��ˮ��
����3���μ�Ũ��ˮ�����裬���ڷ�Ӧ���ʣ��ڷ�Ӧ���еõ���Ʒ
����
�ٸ������ʢ�ŵ�ҩƷ�� ��
�ڶԱ�̼���κ��ᷴӦ��CO2����ʵ�����øɱ���������CO2������ŵ��� ��
���Ժ�ѹ��Һ©�������Һ©����Ŀ���� ��
�ܷ�Ӧ��������CO2�������������·�Ӧ������������ɲ�ȡ����Ӧ��ʩ�� ��
��4����ͬѧ��Ϊ��ʵ��װ�ô��ڰ�ȫ���⣬���ʿ������ٵİ�ȫ������ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com