
³£ĪĀĻĀ£¬0.1m01£ÆLµÄĻĀĮŠČÜŅŗ£ŗ
¢ŁHCl”¢¢ŚCH3COOH”¢¢ŪCH3COONa”¢¢ÜNaOH”¢¢ŻFeCl3”¢¢ŽNaCl”£
£Ø1£©pHÓÉŠ”µ½“óÅÅĮŠĖ³ŠņĪŖ £ØĢīŠņŗÅ£©£»
£Ø2£©ŹµŃéŹŅÅäÖĘ¢ŻµÄČÜŅŗŹ±³£Šč¼ÓČėÉŁĮæŃĪĖį£¬·ńŌņµĆµ½µÄŹĒ»ė×ĒµÄČÜŅŗ£¬²śÉś»ė×ĒµÄŌŅņŹĒ£ØÓĆĄė×Ó·½³ĢŹ½±ķŹ¾£©
£Ø3£©ĪļÖŹµÄĮæÅضČĻąĶ¬µÄ¢Ł°±Ė®¢ŚĀČ»Æļ§¢ŪĢ¼ĖįĒāļ§¢ÜĮņĖįĒāļ§¢ŻĮņĖįļ§
ŌŚÉĻŹöĪåÖÖČÜŅŗÖŠ£¬ļ§øłĄė×ÓĪļÖŹµÄĮæÅضČÓɓ󵽊”µÄĖ³ŠņŹĒ
£Ø4£©ŅŃÖŖ²šæŖ1molH”ŖH¼ü£¬1molN”ŖH¼ü£¬1molN”ŌN¼ü·Ö±šŠčŅŖµÄÄÜĮæŹĒ436kJ”¢39l kJ”¢946 kJ£¬ŌņN2ÓėH2·“Ӧɜ³ÉNH3µÄČČ»Æѧ·½³ĢŹ½ĪŖ
£Ø1£©¢Ł¢Ś¢Ż¢Ž¢Ū¢Ü £Ø2£©Fe3++3H2O Fe(OH)3+3H+
Fe(OH)3+3H+
£Ø3£©¢Ż£¾¢Ü£¾¢Ś£¾¢Ū£¾¢Ł £Ø4£©N2(g)+3H2(g) 2NH3(g)
2NH3(g) 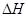 =-92.0kJ/mol
=-92.0kJ/mol
½āĪöŹŌĢā·ÖĪö£ŗ£Ø1£©ŃĪĖįŹĒŅ»ŌŖĒæĖį£¬“×ĖįŹĒŅ»ŌŖČõĖį£¬“×ĖįÄĘŹĒČõĖįĒæ¼īŃĪ£¬ĒāŃõ»ÆÄĘŹĒŅ»ŌŖĒæ¼ī”£ĀČ»ÆĢśŹĒĒæĖįČõ¼īŃĪ£¬ĀČ»ÆÄĘŹĒĒæĖįĒæ¼īŃĪ£¬ĖłŅŌŌŚÅضČĻąµČµÄĢõ¼žĻĀ£¬pHÓÉŠ”µ½“óÅÅĮŠĖ³ŠņĪŖ¢Ł¢Ś¢Ż¢Ž¢Ū¢Ü”£
£Ø2£©ĀČ»ÆĢśČÜÓŚĖ®£¬ĢśĄė×ÓĖ®½āÉś³ÉĒāŃõ»ÆĢśŗĶŃĪĖį£¬ĖłŅŌ¼ÓČėŃĪĖįµÄÄæµÄŹĒŅÖÖĘĢśĄė×ÓµÄĖ®½ā£¬·½³ĢŹ½ŹĒFe3++3H2O Fe(OH)3+3H+”£
Fe(OH)3+3H+ӣ
£Ø3£©ŌŚČÜŅŗÖŠNH4£«Ė®½ā£¬¶ųĮņĖįĒāļ§ÄܵēĄė³öĒāĄė×Ó£¬ŅÖÖĘNH4£«Ė®½ā”£HCO3£Ė®½ā£¬ĻŌ¼īŠŌ£¬“Ł½ųNH4£«Ė®½ā£¬ĖłŅŌĪåÖÖČÜŅŗÖŠ£¬ļ§øłĄė×ÓĪļÖŹµÄĮæÅضČÓɓ󵽊”µÄĖ³ŠņŹĒ¢Ż£¾¢Ü£¾¢Ś£¾¢Ū£¾¢Ł”£
£Ø4£©·“Ó¦ČČŹĒ¶Ļ¼üĪüŹÕµÄÄÜĮæŗĶŠĪ³É»Æѧ¼üĖł·Å³öµÄÄÜĮæµÄ²īÖµ£¬ŌņøĆ·“Ó¦µÄ·“Ó¦ČČ”÷H£½3”Į436kJ/mol£«946kJ/mol£2”Į3”Į391kJ/mol£½£92.0kJ/mol£¬ĖłŅŌ·“Ó¦µÄČČ»Æѧ·½³ĢŹ½ŹĒN2(g)+3H2(g) 2NH3(g)
2NH3(g) 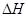 =-92.0kJ/molӣ
=-92.0kJ/molӣ
æ¼µć£ŗæ¼²épHµÄÅŠ¶Ļ”¢ŃĪĄąĖ®½āµÄÓ¦ÓĆ”¢ČÜŅŗÖŠĄė×ÓÅØ¶Č±Č½ĻŅŌ¼°·“Ó¦ČČµÄ¼ĘĖć
µćĘĄ£ŗøĆĢāŹĒøßæ¼ÖŠµÄ³£¼ūĢāŠĶ£¬ŹōÓŚÖŠµČÄѶȵďŌĢā”£ŹŌĢā×ŪŗĻŠŌĒ棬²īÖµ¶ŌѧɜĮé»īŌĖÓĆ»ł“”ÖŖŹ¶½ā¾öŹµ¼ŹĪŹĢāµÄÄÜĮæµÄÅąŃų”£Ö¼ŌŚĢįøßѧɜ·ÖĪö”¢¹éÄÉ”¢×ܽįĪŹĢāµÄÄÜĮ¦”£ÓŠĄūÓŚµ÷¶ÆѧɜµÄѧĻ°ŠĖȤŗĶѧĻ°»ż¼«ŠŌ£¬Ņ²ÓŠÖśÓŚÅąŃųѧɜµÄĀß¼ĶĘĄķÄÜĮ¦ŗĶ³éĻóĖ¼Ī¬ÄÜĮ¦”£



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2011-2012ѧğø£½ØŹ”ÉĻŗ¼Ņ»ÖŠø߶žĻĀŃ§ĘŚĘŚÄ©æ¼ŹŌ»ÆѧŹŌ¾ķ£Ø“ų½āĪö£© ĢāŠĶ£ŗĢīæÕĢā
£Ø8·Ö£¬ĆææÕøń2·Ö£©³£ĪĀĻĀ£¬0.1m01£ÆLµÄĻĀĮŠČÜŅŗ£ŗ
¢ŁHCl”¢¢ŚCH3COOH”¢¢ŪCH3COONa”¢¢ÜNaOH”¢¢ŻFeCl3”¢¢ŽNaCl”£
£Ø1£©pHÓÉŠ”µ½“óÅÅĮŠĖ³ŠņĪŖ £ØĢīŠņŗÅ£©£»
£Ø2£©ŹµŃéŹŅÅäÖĘ¢ŻµÄČÜŅŗŹ±³£Šč¼ÓČėÉŁĮæŃĪĖį£¬·ńŌņµĆµ½µÄŹĒ»ė×ĒµÄČÜŅŗ£¬²śÉś»ė×ĒµÄŌŅņŹĒ£ØÓĆĄė×Ó·½³ĢŹ½±ķŹ¾£©
£Ø3£©ĪļÖŹµÄĮæÅضČĻąĶ¬µÄ¢Ł°±Ė®¢ŚĀČ»Æļ§¢ŪĢ¼ĖįĒāļ§¢ÜĮņĖįĒāļ§¢ŻĮņĖįļ§
ŌŚÉĻŹöĪåÖÖČÜŅŗÖŠ£¬ļ§øłĄė×ÓĪļÖŹµÄĮæÅضČÓɓ󵽊”µÄĖ³ŠņŹĒ
£Ø4£©ŅŃÖŖ²šæŖ1molH”ŖH¼ü£¬1molN”ŖH¼ü£¬1molN”ŌN¼ü·Ö±šŠčŅŖµÄÄÜĮæŹĒ436kJ”¢39lkJ”¢946kJ£¬ŌņN2ÓėH2·“Ӧɜ³ÉNH3µÄČČ»Æѧ·½³ĢŹ½ĪŖ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2014½ģŗÓÄĻĮ鱦µŚČżøß¼¶ÖŠŃ§ø߶žĻĀѧʌµŚČż“Ī¼ģ²ā»Æѧ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
³£ĪĀĻĀ£¬0.1m01£ÆLµÄĻĀĮŠČÜŅŗ£ŗ
¢ŁHCl”¢¢ŚCH3COOH”¢¢ŪCH3COONa”¢¢ÜNaOH”¢¢ŻFeCl3”¢¢ŽNaCl”£
£Ø1£©pHÓÉŠ”µ½“óÅÅĮŠĖ³ŠņĪŖ £ØĢīŠņŗÅ£©£»
£Ø2£©ŹµŃéŹŅÅäÖĘ¢ŻµÄČÜŅŗŹ±³£Šč¼ÓČėÉŁĮæŃĪĖį£¬·ńŌņµĆµ½µÄŹĒ»ė×ĒµÄČÜŅŗ£¬²śÉś»ė×ĒµÄŌŅņŹĒ£ØÓĆĄė×Ó·½³ĢŹ½±ķŹ¾£©
£Ø3£©ĪļÖŹµÄĮæÅضČĻąĶ¬µÄ¢Ł°±Ė®¢ŚĀČ»Æļ§¢ŪĢ¼ĖįĒāļ§¢ÜĮņĖįĒāļ§¢ŻĮņĖįļ§
ŌŚÉĻŹöĪåÖÖČÜŅŗÖŠ£¬ļ§øłĄė×ÓĪļÖŹµÄĮæÅضČÓɓ󵽊”µÄĖ³ŠņŹĒ
£Ø4£©ŅŃÖŖ²šæŖ1molH”ŖH¼ü£¬1molN”ŖH¼ü£¬1molN”ŌN¼ü·Ö±šŠčŅŖµÄÄÜĮæŹĒ436kJ”¢39l kJ”¢946 kJ£¬ŌņN2ÓėH2·“Ӧɜ³ÉNH3µÄČČ»Æѧ·½³ĢŹ½ĪŖ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2014½ģ½Ī÷Ź”ø߶žµŚ¶ž“ĪŌĀæ¼»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
£Ø15·Ö£©£Ø¢ń£©ŌŚĪĀ¶Čt ”ꏱ£¬pH=3µÄijĖ®ČÜŅŗÖŠc(OH£)=10£9 mol£ÆLŌŚ“ĖĪĀ¶ČĻĀpH¾łĪŖ4µÄŃĪĖįŗĶ(NH4)2SO4ČÜŅŗÖŠÓÉĖ®µēĄė³öµÄc(H+)Ö®±ČĪŖ__________”£
£Ø¢ņ£©³£ĪĀĻĀ£¬pH=10µÄĒæ¼īAOHŗĶpH=4µÄĖįHnBµČĢå»ż»ģŗĻŗóČÜŅŗĻŌĖįŠŌ£¬Éś³ÉµÄŃĪ»ÆѧŹ½ĪŖ___________”£AOHÓėHnBĶźČ«ÖŠŗĶĖłµĆČÜŅŗ³Ź_________ŠŌ£¬ĘäŌŅņÓĆĄė×Ó·½³ĢŹ½±ķ_______________________________________”£
£Ø¢ó£©³£ĪĀĻĀ½«Ģå»żĪŖv1”¢ÅضČĪŖc1µÄŅ»ŌŖĖįHAÓėĢå»żĪŖv2”¢ÅضČĪŖc2µÄŅ»ŌŖ¼īBOH»ģŗĻ”£
(1)Čōv1”¤c1£½v2”¤c2£¬»ģŗĻŗóµÄČÜŅŗpH£¾7£¬ŌņŅ»ŌŖČõĖįµÄµēĄė³Ģ¶Č £ØŃ”Ģī”°£¾”±”¢”°£¼”±»ņ”°£½”±£©Ņ»ŌŖČõ¼īµÄµēĄė³Ģ¶Č£»
(2)Čōv1£½v2£¬Ņ»ŌŖČõ¼īµÄµēĄė³Ģ¶Č“óÓŚŅ»ŌŖČõĖįµÄµēĄė³Ģ¶Č£¬ĒŅ»ģŗĻŗóČÜŅŗpH£¼7£¬Ōņc1 c2£ØŃ”Ģī”°£¾”±”¢”°£¼”±»ņ”°£½”±£©”£
£Ø¢ō£©³£ĪĀĻĀ£¬0.1m01£ÆLµÄĻĀĮŠČÜŅŗ£ŗ
¢ŁHCl”¢¢ŚCH3COOH”¢¢ŪCH3COONa”¢¢ÜNaOH”¢¢ŻFeCl3”¢¢ŽNaCl”£
£Ø1£©pHÓÉŠ”µ½“óÅÅĮŠĖ³ŠņĪŖ £ØĢīŠņŗÅ£©£»
£Ø2£©ŹµŃéŹŅÅäÖĘ¢ŻµÄČÜŅŗŹ±³£Šč¼ÓČėÉŁĮæŃĪĖį£¬·ńŌņµĆµ½µÄŹĒ»ė×ĒµÄČÜŅŗ£¬²śÉś»ė×ĒµÄŌŅņŹĒ£ØÓĆĄė×Ó·½³ĢŹ½±ķŹ¾£©
£Ø¢õ£©½«ĮņĖįĀĮČÜŅŗŗĶĢ¼ĖįĒāÄĘČÜŅŗ»ģŗĻŹ±£¬æÉ擵½µÄŹµŃéĻÖĻóŹĒ ,²śÉśøĆĻÖĻóµÄŌŅņŹĒ £ØÓĆĄė×Ó·½³ĢŹ½½āŹĶŌŅņ£©”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2013½ģø£½ØŹ”ø߶žĻĀŃ§ĘŚĘŚÄ©æ¼ŹŌ»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
£Ø8·Ö£¬ĆææÕøń2·Ö£©³£ĪĀĻĀ£¬0.1m01£ÆLµÄĻĀĮŠČÜŅŗ£ŗ
¢ŁHCl”¢¢ŚCH3COOH”¢¢ŪCH3COONa”¢¢ÜNaOH”¢¢ŻFeCl3”¢¢ŽNaCl”£
£Ø1£©pHÓÉŠ”µ½“óÅÅĮŠĖ³ŠņĪŖ £ØĢīŠņŗÅ£©£»
£Ø2£©ŹµŃéŹŅÅäÖĘ¢ŻµÄČÜŅŗŹ±³£Šč¼ÓČėÉŁĮæŃĪĖį£¬·ńŌņµĆµ½µÄŹĒ»ė×ĒµÄČÜŅŗ£¬²śÉś»ė×ĒµÄŌŅņŹĒ£ØÓĆĄė×Ó·½³ĢŹ½±ķŹ¾£©
£Ø3£©ĪļÖŹµÄĮæÅضČĻąĶ¬µÄ¢Ł°±Ė®¢ŚĀČ»Æļ§¢ŪĢ¼ĖįĒāļ§¢ÜĮņĖįĒāļ§¢ŻĮņĖįļ§
ŌŚÉĻŹöĪåÖÖČÜŅŗÖŠ£¬ļ§øłĄė×ÓĪļÖŹµÄĮæÅضČÓɓ󵽊”µÄĖ³ŠņŹĒ
£Ø4£©ŅŃÖŖ²šæŖ1molH”ŖH¼ü£¬1molN”ŖH¼ü£¬1molN”ŌN¼ü·Ö±šŠčŅŖµÄÄÜĮæŹĒ436kJ”¢39lkJ”¢946kJ£¬ŌņN2ÓėH2·“Ӧɜ³ÉNH3µÄČČ»Æѧ·½³ĢŹ½ĪŖ
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com