
����֬�ް�סԼ0.2g�������Ʒ�ĩ������ʯ�����ϣ�����֬���ϵ�ˮ��
�۲쵽��֬����ȼ��������
(1)������ʵ���������ó����йع������Ƹ�ˮ��Ӧ�Ľ����ǣ���һ�����������ɣ��ڶ���_________�� ��ˮ��Ӧ�Ļ�ѧ����ʽ��
��ˮ��Ӧ�Ļ�ѧ����ʽ��
___________�����л�ԭ����____________����������_____________��
(2)ij�о���ѧϰС������ͼ��ʾװ��(����������)����ʵ�飬��֤���������ۣ�������֤��һ�����۵�ʵ�鷽���ǣ�_____________________��
������֤�ڶ������۵�ʵ�鷽���ǣ�__________________________��

(3)ʵ��(2)���Թ��м�ˮ��������ȫ�ܽ��Ҳ������������ɺ�ȡ���Թܣ����Թ��е����̪��Һ��������Һ�ȱ�����ɫ��Ϊ̽����ԭ��С��ͬѧ�����й����ϵ�֪�� ��ˮ��Ӧ������
��ˮ��Ӧ������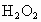 ��
��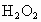 ����ǿ�����Ժ�Ư���ԣ������һ����ʵ�飬��֤
����ǿ�����Ժ�Ư���ԣ������һ����ʵ�飬��֤ ������ˮ��ַ�Ӧ�����Һ����
������ˮ��ַ�Ӧ�����Һ����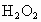 ���ڣ�(ֻҪ��д��ʵ�����õ��Լ����۲쵽������)
���ڣ�(ֻҪ��д��ʵ�����õ��Լ����۲쵽������)
�Լ���_______________________________________��
����_______________________________________��
(4)��С��ͬѧ����ö����ķ���̽�� ��ˮ��Ӧ�����Һ���Ƿ���
��ˮ��Ӧ�����Һ���Ƿ���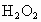 ����ʵ�鷽��Ϊ����ȡ2.6g
����ʵ�鷽��Ϊ����ȡ2.6g ���壬ʹ֮��������ˮ��Ӧ����������
���壬ʹ֮��������ˮ��Ӧ���������� �������������ֵ�Ƚϣ����ɵó����ۣ�
�������������ֵ�Ƚϣ����ɵó����ۣ�

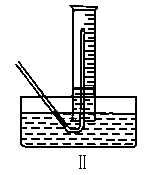
�ٲ����������ʱ��������Թܺ���Ͳ�ڵ����嶼��ȴ������ʱ���У�Ӧѡ����ͼװ���е�(���Ե�������Ͳ����ռ�����)________(�����)��������____________��
�����ڱ�״���²�������������Ӧѡ�õ���Ͳ�Ĵ�С���Ϊ________(ѡ�100mL������200mL������500mL����1000mL��)��
 ����Ӣ��ϵ�д�
����Ӣ��ϵ�д� ����ѧУ�ֲ����ܲ�ϵ�д�
����ѧУ�ֲ����ܲ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��1����ʵ���������ó����й�Na2O2��H2O��Ӧ�Ľ����ǣ�a.���������ɣ�b. ��Na2O2��H2O��Ӧ�Ļ�ѧ����ʽ�ǣ� ��
��2��ij�о���ѧϰС��������ͼװ�ý���ʵ�飬��֤���������ۡ�������֤����a��ʵ�鷽���ǣ� ��������֤����b��ʵ�鷽���������ǣ� ��

��3��ʵ�飨2�������Թ��ڼ�ˮ��������ȫ�ܽ��Ҳ������������ɺ�ȡ���Թܣ����Թ��е����̪��Һ��������Һ��죬��ɫ���ʡ�Ϊ̽��������С��ͬѧ�����й����ϵ�֪��Na2O2��H2O��Ӧ������H2O2��H2O2����ǿ�����Ժ�Ư���ԡ������һ����ʵ�飬֤��Na2O2������H2O��ַ�Ӧ�����Һ����H2O2���ڣ�ֻҪ���г�ʵ�����õ��Լ����۲쵽������
�Լ��� ��
���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(1)������ʵ������ɵó������йع������Ƹ�ˮ��Ӧ�Ľ����ǣ�
��һ�����������ɣ��ڶ���_____________________������������ˮ�ķ�Ӧ�У���ԭ���ǣ�_______________________��
ͼ2-1
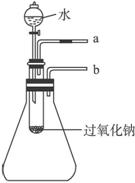
(2)ij�о���ѧϰС��������ͼ21��ʾװ�ý���ʵ�飬��֤���������ۡ�
������֤��һ�����۵�ʵ�鷽����������_____________________________________��
������֤�ڶ������۵�ʵ�鷽����������_____________________________________��
(3)ʵ��(2)���Թ��м�ˮ��������ȫ�ܽ��Ҳ������������ɺ�ȡ���Թܣ����Թ��е���ʯ����Һ��������Һ����������ɫ��ȥ��Ϊ̽��������С��ͬѧ�Ӳ����й������е�֪�������Ƹ�ˮ��Ӧ�����ɹ������⣬�����������ǿ�����Ժ�Ư���ԡ������һ����ʵ�飬֤���������ƺ�����ˮ��ַ�Ӧ�����Һ���й���������ڡ�����������Һ������������Һ������������Һ���⻯����Һ����ɫ�����ȣ������ѡ���Լ���������֤��(ֻҪ���г�ʵ�����õ��Լ����۲쵽������)
�Լ���________________________________________________________________;
����________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��1��������ʵ���������ó����йع������Ƹ�ˮ��Ӧ�Ľ����ǣ���һ�����������ɣ��ڶ���____________���������Ƹ�ˮ��Ӧ�Ļ�ѧ����ʽ��________________________�����л�ԭ����________________________��
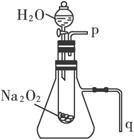
��2��ij�о���ѧϰС��������ͼװ�ý���ʵ�飬��֤���������ۡ�
������֤��һ�����۵�ʵ�鷽���ǣ�______________________________��
������֤�ڶ������۵�ʵ�鷽���������ǣ�_______________________________________��
��3��ʵ�飨2�������Թ��ڼ�ˮ��������ȫ�ܽ��Ҳ������������ɺ�ȡ���Թܣ����Թ��е����̪��Һ��������Һ��죻��ɫ��ȥ��Ϊ̽��������С��ͬѧ�����й����ϵ�֪��Na2O2��H2O��Ӧ������H2O2��H2O2����ǿ�����Ժ�Ư���ԡ������һ����ʵ�飬֤��Na2O2������H2O��ַ�Ӧ�����Һ����H2O2���ڡ�����Na2S��Һ��KI��Һ����ɫ�����ȣ������ѡ���Լ���������֤��ֻҪ���г�ʵ�����õ��Լ����۲쵽������
�Լ���______________________________________________________________��
����______________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

��1����ʵ���������ó����й�Na2O2��H2O��Ӧ�Ľ�����:a.���������ɣ�b._________��
��2��д��Na2O2��H2O��Ӧ�Ļ�ѧ����ʽ����������ת����Ŀ: ___________________��
��3��ijѧУ�о���ѧϰС��������ͼװ�ý���ʵ�飬��֤���������ۡ�
��������֤����a��ʵ�����������������_____________________________________��
��������֤����b��ʵ�����������������_____________________________________��
��4�����о���ѧϰС���ͬѧ��ΪNa2O2��H2O��Ӧ������H2O2�����������һ����ʵ����֤��Na2O2��������H2O��ַ�Ӧ�����Һ����H2O2���ڡ���ֻҪ���г�ʵ�����õ��Լ����۲쵽������
�Լ���______________________________________________________________��
����______________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ������ʡ�����и����ص�����ѧ��һ�������¿���ѧ�Ծ����������� ���ͣ������
����֬�ް�סԼ0.2 g�������Ʒ�ĩ������ʯ�����ϣ�����֬���ϵ�ˮ���ɹ۲쵽��֬����ȼ��������
��1��������ʵ���������ó����йع������Ƹ�ˮ��Ӧ�Ľ����ǣ���һ�����������ɣ��ڶ���____________ ��
�������Ƹ�ˮ��Ӧ�Ļ�ѧ����ʽ��________________________��
��2��ij�о���ѧϰС��������ͼװ�ý���ʵ�飬��֤���������ۡ�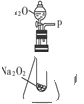
������֤��һ�����۵�ʵ�鷽���ǣ�__________________ ��
������֤�ڶ������۵�ʵ�鷽���������ǣ�_______________________________��
��3��ʵ�飨2�������Թ��ڼ�ˮ��������ȫ�ܽ��Ҳ������������ɺ�ȡ���Թܣ����Թ��е����̪��Һ��������Һ��죻��ɫ��ȥ��Ϊ̽��������С��ͬѧ�����й����ϵ�֪��Na2O2��H2O��Ӧ������H2O2��H2O2����ǿ�����Ժ�Ư���ԡ������һ����ʵ�飬֤��Na2O2������H2O��ַ�Ӧ�����Һ����H2O2���ڡ�����Na2S��Һ��KI��Һ����ɫ�����ȣ������ѡ���Լ���������֤��ֻҪ���г�ʵ�����õ��Լ����۲쵽������
�Լ���____________ ������______________ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com