��2012?��ƽ��һģ����Ƴ������������ƹ��գ����������Ƶķ�ˮʱ�����ڴ���TiO
2�����£�����NaClO��CN
-����������CNO
-���������������¼�����NaClO������N
2��CO
2������������Ա���ܱ�ϵͳ������ͼװ�ý���ʵ�飬��֤��������������Ч�ԣ���ͨ���ⶨ������̼����ȷ����CN
-�������İٷ��ʣ�
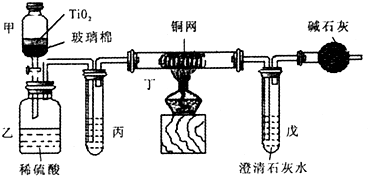
��Ũ����CN
-���ӵ���ˮ�����NaClO��Һ�Ļ��Һ200mL������CN
-��Ũ��0.05mol/L��������У�������Ƥ����һ��ʱ�����Ƥ���ͻ�����ʹ��Һȫ���������У��رջ������ش��������⣺
��1�����з�Ӧ�����ӷ���ʽΪ
CN-+ClO-�TCNO-+Cl-
CN-+ClO-�TCNO-+Cl-
��
���з�Ӧ�����ӷ���ʽΪ
2CNO-+2H++3ClO-=N2��+2CO2��+3Cl-+H2O
2CNO-+2H++3ClO-=N2��+2CO2��+3Cl-+H2O
��
��2���������ɵ������N
2��CO
2�⣬����HCl��������Cl
2�ȣ����м���ij����Լ��DZ���ʳ��ˮ����������
��ȥHCl����
��ȥHCl����
����ʵ���е�������
ȥ��Cl2
ȥ��Cl2
��װ�м�ʯ�ҵĸ���ܵ�������
��ֹ������CO2��������Ӱ��ⶨȷ��
��ֹ������CO2��������Ӱ��ⶨȷ��
��
��3������ʢ�к�Ca��OH��
2 0.02mol��ʯ��ˮ����ʵ�������й�����0.82g���������ʵ���в��CN
-�������İٷ��ʵ���
82%
82%
���ò��ֵ�빤ҵʵ�ʴ����İٷ����������ƫ�ͣ���Ҫ˵�����ܵ�ԭ��
��װ���ҡ��������п���������CO2����CO2�������ٶȽϿ�δ�����ж�����ʯ��ˮ��ַ�Ӧ����Cl2��HCl�ڱ�������δ������ȫ����������������㼴�ɣ�
��װ���ҡ��������п���������CO2����CO2�������ٶȽϿ�δ�����ж�����ʯ��ˮ��ַ�Ӧ����Cl2��HCl�ڱ�������δ������ȫ����������������㼴�ɣ�
�����ٴ�����ԭ�������һ�������ȷ�ȵĽ��飨Ҫ�пɲ����ԣ��Ҳ�����
����һ������������Һһ��ȫ���������У���Ϊ�ִμ��룬����CO2�IJ����ٶȣ�
�����������ƿ����Ϊ�����������Ӷ��Ǹ����в���һ�����ܵ�Һ�����£���Ӧ������ͨ���ȥCO2�Ŀ�����ʹװ����������CO2�����ܶൽ��Ca��OH��2��Ӧ��
�������������г���ʯ��ˮ��ΪŨ�Ƚϴ�NaOH��Һ����Ӧ���������м�������CaCl2�����������ȵȣ������������һ�㼴�ɣ�
����һ������������Һһ��ȫ���������У���Ϊ�ִμ��룬����CO2�IJ����ٶȣ�
�����������ƿ����Ϊ�����������Ӷ��Ǹ����в���һ�����ܵ�Һ�����£���Ӧ������ͨ���ȥCO2�Ŀ�����ʹװ����������CO2�����ܶൽ��Ca��OH��2��Ӧ��
�������������г���ʯ��ˮ��ΪŨ�Ƚϴ�NaOH��Һ����Ӧ���������м�������CaCl2�����������ȵȣ������������һ�㼴�ɣ�
��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
