
| c2(NH3) |
| c3(H2)c(N2) |
| c2(NH3) |
| c3(H2)c(N2) |
| 3��0.3 |
| 3 |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| ||
| ||
| �� |
| ||
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| �� |
| ||
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
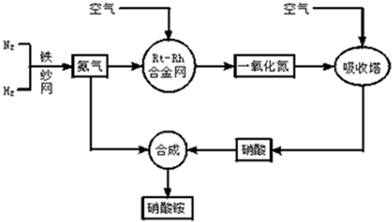
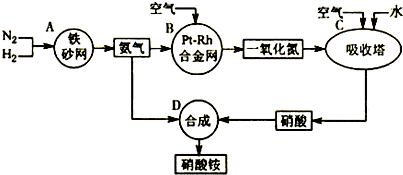
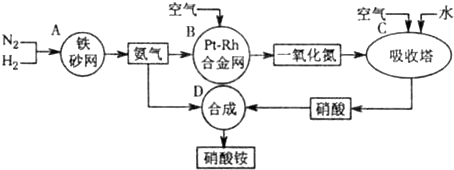
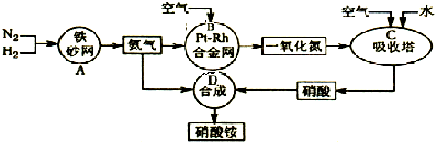
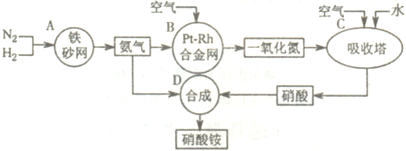
��10�֣���ͼ�ǹ�ҵ��������淋����̡�
��1��������C��ͨ�������Ŀ���� ��
A��B��C��D�ĸ������еķ�Ӧ������������ԭ��Ӧ���� ������ĸ����
��2����֪��4NH3��g��+3O2��g��=2N2��g��+6H2O��g�� ��H = ��1266��8kJ��mol
N2��g��+O2��g��=2NO��g�� ��H= +180��5 kJ��mol
д�������´��������Ȼ�ѧ����ʽ�� ������������Ӧ�Ļ�ѧƽ�ⳣ������ʽK= ��
��3����֪�� ��H= �� 92 kJ��mol��Ϊ���������ת���ʣ��˲�ȡ�Ĵ�ʩ�� ��������ĸ��
A�������¶� B��ʹ�ô��� C������ѹǿ
D��ѭ�����úͲ��ϲ��䵪�� E����ʱ�Ƴ���
��4����һ���¶Ⱥ�ѹǿ�£���H2��N2��3��1������ȣ����ܱ������л�ϣ����÷�Ӧ�ﵽƽ��ʱ�����ƽ��������NH3�������������Ϊ17��6������ʱH2��ת����Ϊ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com