
| ||
| 2.52g |
| 126g?mol-1 |
| ||


 53���ò�ϵ�д�
53���ò�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪�Ҷ��ᣨHOOC�DCOOH���׳Ʋ��ᣬ������ˮ��Ϊ��Ԫ���ᣬ����ǿ��̼�ᣬ���β���ƺͲ�����ƾ�Ϊ��ɫ�������ɫ����H2C2O4?2H2O��Ϊ���ᾧ�壬���۵�Ϊ101.5�档���ᾧ��ʧȥ�ᾧˮ����ˮ���ᣬ����157��������
����������Ϣ���ش��������⡣
��1����ʢ��2mL����NaHCO3��Һ���Թ������Լ2mL�Ҷ���Ũ��Һ���۲쵽��������________��д���÷�Ӧ�����ӷ���ʽ��___________________��
��2����ƽ�Ҷ���������KMnO4��Һ��Ӧ�����ӷ���ʽ����ѧ����������������Ӧ�ķ����ڣ���
_____MnO4�� +_____H2C2O4 +_____H+ _____Mn2+ +_____CO2��+_____H2O
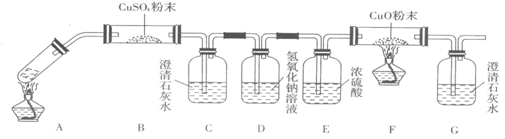
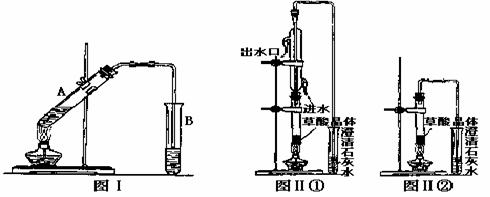
��3�����Թ�A�м���3mL�Ҵ���Ȼ������Թܱ���2mLŨ�����2mL�Ҷ�����Һ������ͼ�����Ӻ�װ�ã�����3�D5min����װ�б���Na2CO3��Һ���Թ�B������״�Ҵ�����ζ��Һ�������B�е��ܿ���Һ���϶�������Һ���µ�ԭ����__________________��д���Ҷ����������Ҵ���ȫ�����Ļ�ѧ��Ӧ����ʽ__________________��

��4����֪����ֽ�Ļ�ѧ����ʽΪ��H2C2O4![]() H2O+CO2��+CO����
H2O+CO2��+CO����
����ͼ��ٺ�ͼ��ڼ��Ȳ��ᾧ�壬����֤���������Ƿ�ֽ⡣��������һ��ʱ���������ǣ�ͼ��٣��Թ������ʯ��ˮ�ȱ���ǣ����ֱ���壬��ԭ����___________��
ͼ��ڣ��Թ������ʯ��ˮֻ����ǣ���ԭ����__________������֤�������ȷֽ��װ����______����ͼ���еı�� ���١��ڡ����������ǣ�___________(��װ�õ��ص����)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪�Ҷ��ᣨ HOOC��COOH���׳Ʋ��ᣬ����һ��������ˮ�Ķ�Ԫ���ᣬ����ǿ��̼�ᣬ���β���ƺͲ�����ƾ�Ϊ��ɫ�������ɫ����H2C2O4��2H2O��Ϊ���ᾧ�壬���۵�Ϊ101.5�档���ᾧ��ʧȥ�ᾧˮ����ˮ���ᣬ����157��������
����������Ϣ���ش��������⡣
��1����ʢ��2mL����NaHCO3��Һ���Թ������Լ2mL�Ҷ���Ũ��Һ���۲쵽�������� ��д���÷�Ӧ�����ӷ���ʽ�� ��
��2����ƽ�Ҷ���������KMnO4��Һ��Ӧ�����ӷ���ʽ��
MnO4�� + H2C2O4 + H+ �� Mn2+ + CO2��+ H2O
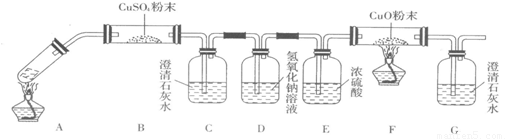
��3�����Թ�A�м���3mL�Ҵ���Ȼ������Թܱ���2mLŨ�����2mL�Ҷ�����Һ������ͼ�����Ӻ�װ�ã�����3��5min����װ�б���Na2CO3��Һ���Թ�B������״�Ҵ�����ζ��Һ�������B�е��ܿ���Һ���϶�������Һ���µ�ԭ���� ��д���Ҷ����������Ҵ���ȫ�����Ļ�ѧ��Ӧ����ʽ ��
��4����֪����ֽ�Ļ�ѧ����ʽΪ��![]() ��
��
����ͼ��ٺ�ͼ��ڼ��Ȳ��ᾧ�壬����֤���������Ƿ�ֽ⡣��������һ��ʱ���������ǣ�ͼ��٣��Թ������ʯ��ˮ�ȱ���ǣ����ֱ���壬��ԭ���� ��ͼ��ڣ��Թ������ʯ��ˮֻ����ǣ���ԭ���� ������֤�������ȷֽ��װ���� ����ͼ���еı�š��١��ڡ����������ǣ� (��װ�õ��ص����)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011�����ʡ������ƽ���к�ѧУ�߿���ѧģ���Ծ���4�·ݣ��������棩 ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com