
������������(FeC2O4��2H2O)���������Լ�����Ӱ�������͵�ز�����������﮵��������ش��������⣺
I����ȤС��Բ�����������ķֽ�������ʵ���̽����̽���ֽ�õ��Ĺ����������Ԫ�صĴ�����ʽ��
��1���������
����һ��___________��??? �������ȫ����FeO ��?????? ��������FeO��Fe����
��2�����ʵ�鷽��֤����������
ʵ�鲽�� | ��������� |
����1�����Թ��м��������������ټ�������????????? ������� | ����Һ��ɫ���Ըı࣬����??????? ���ɣ���֤���������ʴ��� |
����2��������1�еõ�����Һ���ˣ���������ˮϴ����ϴ��Һ��ɫ |
|
����3��ȥ����2�õ������������Թ��У��μ�
|
|
��ѡ�Լ���ϡ���ᡢ���Ƶ���ˮ��0.1mol��L-1CuSO4��Һ��20% KSCN��Һ������ˮ��
����ȤС���������в��ĵ���FeC2O4��2H2O���ȷֽ�ʱ�������������¶ȱ仯����������ͼ��ʾ��д�����ȵ�400��ʱ��FeC2O4��2H2O�������ȷֽ�Ļ�ѧ����ʽΪ��_______________
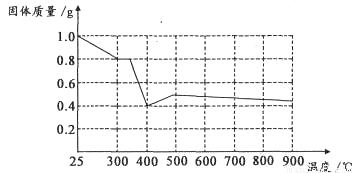
����ͼ������1.0g�������������������г��ڳ�ּ��ȣ����ղ�����ɫ�������������0.4g��ijͬѧ�ɴ˵ó����ۣ�����������������Ƿ�ͬ���ͬѧ�Ľ��ۣ����������ɣ�______________________��????????????
������1��ȫ����Fe
��2��
ʵ�鲽�� | ��������� |
����1������ͭ��Һ | (��)��ɫ���� |
����3������HCl�����ã�ȡ�ϲ���Һ���μӼ���KSCN��Һ���ٵμ��������Ƶ���ˮ ,����� | ���μ��������Ƶ���ˮ����Һ�ʺ�ɫ����֤����FeO |
II��FeC2O4��2H2O FeO+CO��+CO2��+2H2O??????????????????????????????????
FeO+CO��+CO2��+2H2O??????????????????????????????????
��ͬ�⣬ʵ��û�����ܱ������н��У�FeO�ᱻ�����е�������һ��������������������������
��������
���������
����1���������ʵ���ɼ�����ļ������������֪����һΪ����ȫ����Fe����2������һ���������к���FeO��Fe.�����ڽ���Fe�DZȽϻ��õĽ������ܰѻ�Ա������Ľ����û����������Կ�������������м�������ͭ��Һ��������ã���������Һ����ɫ��dz��ͬʱ��������ɫ�Ĺ��塣��֤������Fe���ʡ�������������������õĵĹ�����뵽������ϡHCl�У�������Ӧ��FeO+2HCl=FeCl2+H2O.������ã�ȡ�ϲ���Һ���μӼ���KSCN��Һ���������ٵμ��������Ƶ���ˮ ,���������������Һ��ΪѪ��ɫ����֤�������к���FeO.
II ���������غ㶨�ɺ͵����غ�Ĺ��ɿ�֪FeC2O4��2H2O�������ȷֽ�Ļ�ѧ����ʽΪFeC2O4��2H2O FeO+CO��+CO2��+2H2O�����ݷ���ʽFeC2O4��2H2O
FeO+CO��+CO2��+2H2O�����ݷ���ʽFeC2O4��2H2O FeO+CO��+CO2��+2H2O��֪1g����FeC2O4��2H2O�ֽ������FeO������Ϊ0.4g.�����ڷֽⷴӦ�������г��ڳ�ּ��ȣ�û�����ܱյ������ڽ��С�FeO�л�ԭ�ԣ��ڼ���ʱ���ױ������е���������ΪFe2O3.���Թ���������ܴ���0.4g.��˲����ɴ˵õ�����˵�������������
FeO+CO��+CO2��+2H2O��֪1g����FeC2O4��2H2O�ֽ������FeO������Ϊ0.4g.�����ڷֽⷴӦ�������г��ڳ�ּ��ȣ�û�����ܱյ������ڽ��С�FeO�л�ԭ�ԣ��ڼ���ʱ���ױ������е���������ΪFe2O3.���Թ���������ܴ���0.4g.��˲����ɴ˵õ�����˵�������������
���㣺����ʵ�鷽������������ۡ����ʳɷֵ�ȷ������Ҫ����Fe��Fe3+�ļ��顢����ʽ����д��֪ʶ��


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

 ������õ��˸ߴ��ȵ��������ۣ�
������õ��˸ߴ��ȵ��������ۣ�
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����


c[Cu(NH3
| ||
| c(Cu2+)?c4(NH3) |
| 1 |
| 3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��ij��Һ�н�����������е�5�����ӣ�������ˮ�ĵ��뼰���ӵ�ˮ�⣩�������ӵ����ʵ�����Ϊ1mol��
��ij��Һ�н�����������е�5�����ӣ�������ˮ�ĵ��뼰���ӵ�ˮ�⣩�������ӵ����ʵ�����Ϊ1mol��| ������ | SO42-��NO3-��Cl- |
| ������ | Fe3+��Fe2+��NH4+��Cu2+��Al3+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ʵ�鲽�裨��Ҫ�����IJ������̣� | Ԥ��ʵ������ͽ��� |
| ȡ������ɫ���壬 |
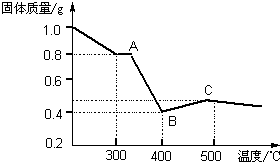 [�����о�]�������������в��ĵ���FeC2O4?2H2O���ȷֽ�ʱ�������������¶ȱ仯��������ͼ��ʾ��д�����ȵ�400��ʱ��FeC2O4?2H2O�������ȷֽ�Ļ�ѧ����ʽΪ��
[�����о�]�������������в��ĵ���FeC2O4?2H2O���ȷֽ�ʱ�������������¶ȱ仯��������ͼ��ʾ��д�����ȵ�400��ʱ��FeC2O4?2H2O�������ȷֽ�Ļ�ѧ����ʽΪ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com