ij���Ը�ʴҺ�к��д���CuCl
2��FeCl
2��FeCl
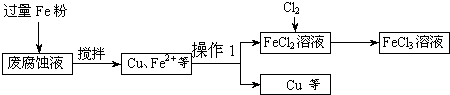
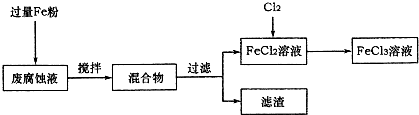
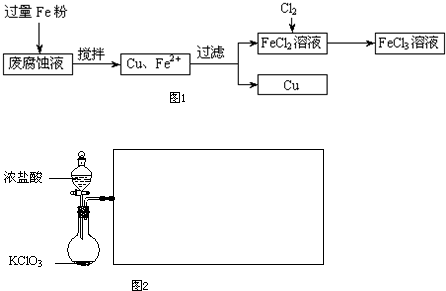
3�������ŷŻ���ɻ�����Ⱦ����Դ���˷ѣ��Էϸ�ʴҺΪԭ�ϣ�����ͭ�������Ļ�����ȫ��ת��ΪFeCl
3��Һ��
���ij���Էϸ�ʴҺ�к�CuCl
2 1.5mol/L��FeCl
2 3mol/L��FeCl
3 1mol/L��HCl 1mol/L��
ȡ�����Էϸ�ʴҺ200mL��������������ʵ���ҽ���ʵ�飺
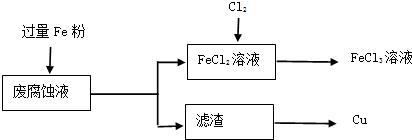
��1����ʴҺ�м������Fe�ۣ�������Ӧ�����ӷ���ʽΪ
��
��2������ϸ�ʴҺ�к���Fe
3+��ʵ�������ȡ�����ϸ�ʴҺ���Թ��У��μӼ���KSCN��Һ����Һ
����֤��ԭ��Һ�к���Fe
3+��
��3����������Ҫ�ɷ���
���ѧʽ�����������õ�ͭ���������Լ���
��
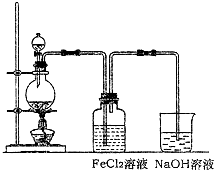
��4��FeCl
2��Һ��ͨ��Cl
2��������Ӧ�Ļ�ѧ����ʽΪ
��
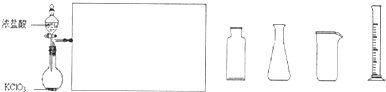
��5��ʵ�����ù���KClO
3��ŨHCl��Ӧ��Cl
2����Ӧ�Ļ�ѧ����ʽΪ��KClO
3+6HCl��Ũ���TKCl+3Cl
2+3H
2O����Ӧ����6.72L����״����Cl
2��ת�Ƶĵ�����Ϊ
mol��
��6�����������̲���������Fe�۵�����Ӧ������
g��