
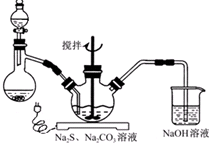
 NaCNΪ�綾���ij��ѧ��ȤС��������ϵ�֪��ʵ��������軯����Һ��ʹ�������������Һ����ͳһ�ⶾ���٣����ǿ�չ����������ʵ�飬����Ҫ��ش����⣺
NaCNΪ�綾���ij��ѧ��ȤС��������ϵ�֪��ʵ��������軯����Һ��ʹ�������������Һ����ͳһ�ⶾ���٣����ǿ�չ����������ʵ�飬����Ҫ��ش����⣺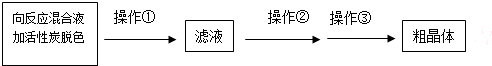
���� ��1�����������ơ����ƺ�̼���Ƶ�Ϊԭ�ϡ���������װ���Ʊ���������ƣ����ݷ�Ӧԭ����֪��������ƿ�м������Ҫʹ��Ӧ���ֽϿ�ķ�Ӧ���ʣ�Ũ���ᡢ���ᶼ�ӷ�����ϡ������룬��Ӧ���ʽ��������������ṩ����Ϣ��֪��Na2S ��Na2CO3�ķ�Ӧ����ʽΪ2Na2S+Na2CO3+4SO2=3Na2S2O3+CO2���ݴ˴��⣻
��2���������⣬Na2S2O3�����������»�����S������Һ��pH�ӽ���С��7ʱ�����SO2ͨ������������NaHSO3���ݴ���д��ѧ����ʽ��
��3����������������Һ�л�ýϸ߲���Na2S2O3?5H2O���ڻ��Һ�м������̼��ɫ��Ȼ����ȹ��ˣ���ֹ��Һ��Na2S2O3?5H2O����������ȥ̼�����Һ��������Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ�����ɵô־��壬�ݴ˴��⣻
��Ʒ���ȵļ��
��1��vmL0.010mol/L��ˮ��Һ��n��I2��=v��10-3L��0.010mol/L=v��10-5mol�����ݹ�ϵʽ2Na2 S2O3��I2���� mg��Ʒ��n��Na2 S2O3��������m=nM����mg��Ʒ��Na2 S2O3•5H2O������������ݴ˾ݴ˴��ȣ�
��2��A����ƿδ��Na2S2O3��Һ��ϴ����ʵ����ûӰ�죻
B���õ�ˮ�ζ�Na2S2O3��Һ����ƿ����Һ����������ֹͣ�ζ������ж����������ĵ�ˮ�������㣻
C���ζ��յ�ʱ���Ӷ�������ʹ��ȡ����ֵƫ��
D���ζ��ܼ����ڵζ�ǰ�����ݣ��ζ��յ㷢�����ݣ�������ı�Һ�����ƫС��
��������ʵ�������֪�����ɵ���Һ��ʹ FeCl3��Һ����Ѫ��ɫ��˵����SCN-���������ݵ���غ��Ԫ���غ����д���ӷ���ʽ��
��� �⣺��1�����������ơ����ƺ�̼���Ƶ�Ϊԭ�ϡ���������װ���Ʊ���������ƣ����ݷ�Ӧԭ����֪��������ƿ�м������Ҫʹ��Ӧ���ֽϿ�ķ�Ӧ���ʣ�Ũ���ᡢ���ᶼ�ӷ�����ϡ������룬��Ӧ���ʽ�����������70%�����ᣬѡC�����������ṩ����Ϣ��֪��Na2S ��Na2CO3�ķ�Ӧ����ʽΪ2Na2S+Na2CO3+4SO2=3Na2S2O3+CO2������Na2S ��Na2CO3��������ʵ�������2��1��
�ʴ�Ϊ��C��2��1��
��2���������⣬Na2S2O3�����������»�����S������Һ��pH�ӽ���С��7ʱ�����SO2ͨ������������NaHSO3����Ӧ�Ļ�ѧ����ʽΪNa2S2O3+SO2+H2O=2NaHSO3+S����
�ʴ�Ϊ��Na2S2O3+SO2+H2O=2NaHSO3+S����
��3����������������Һ�л�ýϸ߲���Na2S2O3?5H2O���ڻ��Һ�м������̼��ɫ��Ȼ����ȹ��ˣ���ֹ��Һ��Na2S2O3?5H2O����������ȥ̼�����Һ��������Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ�����ɵô־��壬���Բ����ٳ��ȹ��ˣ���Ŀ���ǣ�������Ϊ�˷�ֹ�����ڹ��˵Ĺ�������©�����������²��ʽ��ͣ�������Ϊ�˳�ȥ����̿����Ȳ��������ʣ�������������Ũ������ȴ�ᾧ��
�ʴ�Ϊ��������Ϊ�˷�ֹ�����ڹ��˵Ĺ�������©�����������²��ʽ��ͣ�������Ϊ�˳�ȥ����̿����Ȳ��������ʣ�����Ũ������ȴ�ᾧ��
��Ʒ���ȵļ��
��1��vmL0.010mol/L��ˮ��Һ��n��I2��=v��10-3L��0.010mol/L=v��10-5mol����
2Na2 S2O3��������������I2
2 1
n��Na2 S2O3�� v��10-5mol
����n��Na2 S2O3��=2��v��10-5mol=2v��10-5mol
Na2 S2O3•5H2O���������Ϊ2v��10-5mol��248g/mol=496v��10-5g��
�����Ʒ����Ϊ$\frac{496v��10{\;}^{-5}g}{mg}$��100%=$\frac{0.496v}{m}$%��
�ʴ�Ϊ��$\frac{0.496v}{m}$%��
��2��A����ƿδ��Na2S2O3��Һ��ϴ����ʵ����ûӰ�죬��A����
B���õ�ˮ�ζ�Na2S2O3��Һ����ƿ����Һ����������ֹͣ�ζ������ж����������ĵ�ˮ�������㣬�ᵼ��ʵ����ƫ�ͣ���B��ȷ��
C���ζ��յ�ʱ���Ӷ�������ʹ��ȡ����ֵƫ�����ʹʵ����ƫ��C����
D���ζ��ܼ����ڵζ�ǰ�����ݣ��ζ��յ㷢�����ݣ�������ı�Һ�����ƫС���ᵼ��ʵ����ƫ�ͣ���D��ȷ��
��ѡBD��
��������ʵ�������֪�����ɵ���Һ��ʹ FeCl3��Һ����Ѫ��ɫ��˵����SCN-���������ݵ���غ��Ԫ���غ��֪�����ӷ���ʽΪCN-+S2O32-=SCN-+SO32-��
�ʴ�Ϊ��CN-+S2O32-=SCN-+SO32-��
���� ����ͨ����ȡNa2S2O3•5H2O��ʵ������������������Ʊ���������ơ�����ʵ����������ʴ��ȵļ��㡢�ζ��������ȣ���Ŀ�Ѷ��еȣ���ȷʵ���������Ƽ�������ʵ������ǽ����Ĺؼ��������ֿ�����ѧ���ķ������������������Ӧ����ѧ֪ʶ��������


 �����Ծ���ĩ���100��ϵ�д�
�����Ծ���ĩ���100��ϵ�д� ˫��ͬ������ѵ��ϵ�д�
˫��ͬ������ѵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 120�棬��wg��ȩ������������ȼ�գ������ɲ����ù���Na2O2�����������գ���������wg | |
| B�� | 24gMg��������CO2������ȼ�գ����ɹ�������Ϊ40g | |
| C�� | ��ͬ���ʵ�����Al��Al2O3��Al��OH��3������NaOH��Һ��Ӧ����Һ������� | |
| D�� | ��Fe3O4������Ũ����ķ�Ӧ�У��μӷ�Ӧ��Fe3O4���������Ե�HNO3���ʵ���֮��Ϊ1��10 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
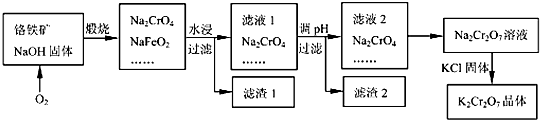
 Cr2O72-+H2O�Ĵ��ڣ���ϡ����������H+��Ũ�ȣ�ƽ�������ƶ���ʹCrO42-Ũ�ȼ�С��Cr2O72-Ũ��������Һ�ɻ�ɫ��Ϊ��ɫ��
Cr2O72-+H2O�Ĵ��ڣ���ϡ����������H+��Ũ�ȣ�ƽ�������ƶ���ʹCrO42-Ũ�ȼ�С��Cr2O72-Ũ��������Һ�ɻ�ɫ��Ϊ��ɫ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | NaOH��Al--����� | B�� | C2H4��O2--��ȼ���� | ||
| C�� | CaC2��K--��ʪ��ȼ��Ʒ | D�� | KMnO4��KClO3--��ԭ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
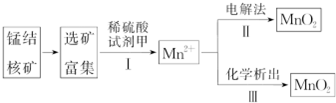
| ʵ�� | 20.0mL˫��ˮ��ҺŨ�� | ��״MnO2 | �¶� | �������� |
| �� | 5% | 2.0g | 20�� | |
| �� | 5% | 1.0g | 20�� | |
| �� | 10% | 1.0g | 20�� | |
| �� | �� | 2.0g | 30�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
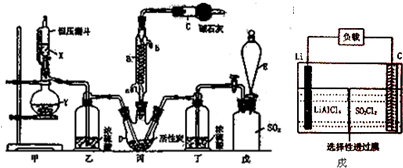
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
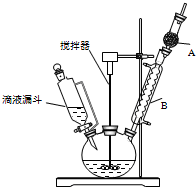 �����״���C6H5��3C-OH��һ����Ҫ�Ļ���ԭ�Ϻ�ҽҩ�м��壮ʵ���Һϳ������״���ʵ��װ����ͼ��ʾ��
�����״���C6H5��3C-OH��һ����Ҫ�Ļ���ԭ�Ϻ�ҽҩ�м��壮ʵ���Һϳ������״���ʵ��װ����ͼ��ʾ��| ���� | �е�/�� |
| �����״� | 380 |
| ���� | 34.6 |
| �屽 | 156.2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
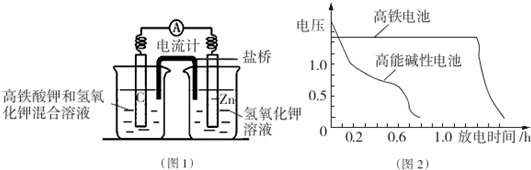
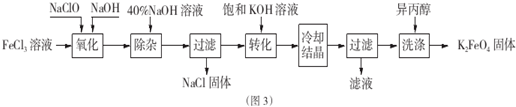
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | NH4C1��Һ��ˮ��������ԣ���NH4C1��������� | |
| B�� | ������Һ��ˮ����Լ��ԣ�ˮ������ӷ���ʽΪ��CO32-+H2O?H2CO3+2OH- | |
| C�� | ������̼��������ԣ����뷽��ʽΪ��H2CO3?CO32-+2H+ | |
| D�� | ����FeC13 ��Һʱ���Ƚ�FeC13 ���ڽ�Ũ�������У�Ȼ���ټ�ˮϡ�͵�����Ũ�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com