
°“ŅŖĒó»Ų“šĻĀĮŠĪŹĢā£ŗ
¢Å³£ĪĀĻĀ£¬ĻņVL 0.1mol/LµÄ“×ĖįČÜŅŗÖŠ¼ÓĖ®Ļ”ŹĶ£¬ĻĀĮŠĖµ·ØÖŠÕżČ·µÄŹĒ £ØĢī×ÖÄø£©
A£®ČÜŅŗÖŠµ¼µēĮ£×ӵďżÄ潫¼õÉŁ B£®ÓÉĖ®µēĄėµÄc(H+)ÅØ¶Č½«¼õŠ”
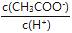
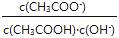
C£®ČÜŅŗÖŠ²»±ä D£®ČÜŅŗÖŠ ½«¼õŠ”
E£®“×ĖįµÄµēĄė³Ģ¶Č½«Ōö“ó£¬c(H+)Ņ²Ōö“ó
¢Ę¢Ł³£ĪĀĻĀ£¬½«0.1mol/LµÄĮņĖįV1mLÓė0.1mol/LNaOHČÜŅŗV2mL»ģŗĻŗó£¬ČÜŅŗµÄpH=1ŌņV1£ŗV2= £ØŗöĀŌČÜŅŗĢå»żµÄ±ä»Æ£©”£
¢Ś³£ĪĀĻĀ£¬ČōČÜŅŗÓÉpH=3µÄŃĪĖįV1mLÓėpH=11µÄij¼īBOHČÜŅŗV2mL»ģŗĻ¶ųµĆ£¬ŌņĻĀĮŠ¼ŁÉčŗĶ½įĀŪ¶¼ÕżČ·µÄŹĒ £ØĢī×ÖÄø£©
A£®Čō»ģŗĻŗóČÜŅŗ³ŹÖŠŠŌ£¬Ōņc(H+)+c(OH-)=2”Į10-7mol/L
B£®ČōV1=V2£¬Ōņ»ģŗĻŗóČÜŅŗµÄpHŅ»¶ØµČÓŚ7
C£®ČōV1=V2£¬Ōņ»ģŗĻŗóŅ»¶ØÓŠ£ŗc(Cl-)£¾c(B+)£¾c(H+)£¾c(OH-)
D£®»ģŗĻŗóµÄČÜŅŗÖŠŅ»¶ØÓŠc(B+)+c(H+)=c(Cl-)+c(OH-)
¢Ē³£ĪĀĻĀ£¬ÅØ¶Č¾łĪŖ0.1mol/LµÄĪåÖÖČÜŅŗµÄpHČēĻĀ±ķĖłŹ¾£ŗ
| ČÜŅŗ | CH3COONa | NaHCO3 | Na2CO3 | NaClO | NaCN |
| pH | 8.8 | 9.7 | 11.6 | 10.3 | 11.1 |
¢ŁŠ“³öĻņNaClOČÜŅŗÖŠĶØČėÉŁĮæCO2µÄĄė×Ó·½³ĢŹ½ ”£
¢Ś½«ÅØ¶Č¾łĪŖ0.01mol/LĻĀĮŠČÜŅŗ·Ö±š¼ÓĖ®Ļ”ŹĶ10±¶£¬pH±ä»Æ×īŠ”µÄŹĒ £ØĢī×ÖÄø£©
A£®HCN B£®HClO C£®H2CO3 D£®CH3COOH
¢Ū³£ĪĀĻĀ£¬µČÅØ¶ČµÄ“×ĖįÓė“×ĖįÄĘ×é³ÉµÄ»ģŗĻČÜŅŗpH=6£¬Ōņc(CH3COO-)£c(CH3COOH)=
(Ģī×¼Č·ŹżÖµ)”£
¢ČŅŃÖŖ³£ĪĀĻĀKsp(AgCl)=1.0”Į10-10£¬Ksp (CH3COOAg)=9.0”Į10-4”£³£ĪĀĻĀ£¬CH3COOAgČōŅŖŌŚNaClČÜŅŗÖŠæŖŹ¼×Ŗ»ÆĪŖAgCl³Įµķ£¬ŌņNaClµÄÅØ¶Č±ŲŠė²»µĶÓŚ ”£

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ(””””)
A£®ČČ»Æѧ·½³ĢŹ½ÖŠ£¬Čē¹ūĆ»ÓŠ×¢Ć÷ĪĀ¶ČŗĶŃ¹Ē棬Ōņ±ķŹ¾·“Ó¦ČČŹĒŌŚ±ź×¼×“æöĻĀ²āµĆµÄ
B£®ÉżøßĪĀ¶Č»ņ¼ÓČė“߻ƼĮ£¬æÉŅŌøıä»Æѧ·“Ó¦µÄ·“Ó¦ČČ
C£®¾ŻÄÜĮæŹŲŗć¶ØĀÉ£¬·“Ó¦ĪļµÄ×ÜÄÜĮæŅ»¶ØµČÓŚÉś³ÉĪļµÄ×ÜÄÜĮæ
D£®ĪļÖŹ·¢Éś»Æѧ±ä»ÆŅ»¶Ø°éĖę×ÅÄÜĮæ±ä»Æ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠĄė×Ó·½³ĢŹ½ÕżČ·µÄŹĒ (”” )
A£®ÅØĻõĖįÖŠ¼ÓČė¹żĮæĢś·Ū²¢¼ÓČČ£ŗFe£«3NO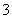 £«6H£«
£«6H£«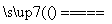 Fe3£«£«3NO2”ü£«3H2O
Fe3£«£«3NO2”ü£«3H2O
B£®Ca(HCO3)2ČÜŅŗÓė¹żĮæNaOHČÜŅŗ·“Ó¦ HCO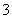 £«OH££«Ca2£«===CaCO3”ż£«H2O
£«OH££«Ca2£«===CaCO3”ż£«H2O
C£®ĒāŃõ»ÆĢśČÜÓŚĒāµāĖįČÜŅŗ£ŗFe(OH)3£«3H£«===Fe3£«£«3H2O
D£®µČĢå»żµČĪļÖŹµÄĮæÅØ¶ČµÄĒāŃõ»Æ±µČÜŅŗÓėĢ¼ĖįĒāļ§ČÜŅŗ»ģŗĻ£ŗ
Ba2£«£«2OH££«NH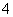 £«HCO
£«HCO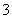 ===BaCO3”ż£«NH3”¤H2O£«H2O
===BaCO3”ż£«NH3”¤H2O£«H2O
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
»ÆŃ§Ę½ŗā³£ŹżKµÄŹżÖµ“󊔏ĒŗāĮæ»Æѧ·“Ó¦½ųŠŠ³Ģ¶ČµÄ±źÖ¾£¬ŌŚ³£ĪĀĻĀ£¬Ä³Š©·“Ó¦ÓėĘ½ŗā³£ŹżŹżÖµČēĻĀ£ŗ2NO(g) N2(g)+O2(g) K1=1”Į1030
N2(g)+O2(g) K1=1”Į1030
2H2(g)+O2(g) 2H2O(g) K2=2”Į1081 2CO2(g)
2H2O(g) K2=2”Į1081 2CO2(g) 2CO(g)+O2(g) K3=4”Į10-92
2CO(g)+O2(g) K3=4”Į10-92
ĻĀĮŠĖµ·ØÖŠÕżČ·µÄŹĒ
A£®³£ĪĀĻĀ£¬Ė®·Ö½ā²śÉśO2£¬“ĖŹ±Ę½ŗā³£ŹżµÄÖµŌ¼ĪŖ5”Į10-80
B£®³£ĪĀĻĀ£¬×īŅ×·Ö½ā·Å³öO2µÄŹĒĖ®
C£®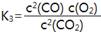 D£®ŅŌÉĻ¶¼²»ÕżČ·
D£®ŅŌÉĻ¶¼²»ÕżČ·
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
¼×±½ŹĒ±½µÄĶ¬ĻµĪļ£¬»ÆѧŠŌÖŹÓė±½ĻąĖĘ£¬µ«ÓÉÓŚ»łĶÅÖ®¼äµÄĻą»„Ó°Ļģ£¬Ź¹¼×±½µÄijŠ©ŠŌÖŹÓÖÓė±½²»Ķ¬”£ĻĀĮŠÓŠ¹ŲĶʶĻ¼°½āŹĶ¶¼ÕżČ·µÄŹĒ
A£®±½²»ÄÜĶعż»Æѧ·“Ó¦Ź¹äåĖ®ĶŹÉ«£¬µ«¼×±½æÉŅŌ£¬ÕāŹĒ¼×»ł¶Ō±½»·Ó°ĻģµÄ½į¹ū
B£®±½²»ÄÜŌŚ¹āÕÕĻĀÓėCl2·¢ÉśČ”“ś·“Ó¦£¬µ«¼×±½æÉŅŌ£¬ÕāŹĒ±½»·¶Ō¼×»łÓ°ĻģµÄ½į¹ū
C£®±½µÄŅ»ĀČ“śĪļÖ»ÓŠŅ»ÖÖ£¬¶ų¼×±½µÄŅ»ĀČ“śĪļÓŠ3ÖÖ£¬ÕāŹĒ±½»·¶Ō¼×»łÓ°ĻģµÄ½į¹ū
D£®±½²»ÄÜŹ¹ĖįŠŌKMnO4ČÜŅŗĶŹÉ«£¬µ«¼×±½æÉŅŌ£¬ÕāŹĒ±½»·¶Ō¼×»łÓ°ĻģµÄ½į¹ū
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
Ķź³ÉĻĀĮŠĄė×Ó·½³ĢŹ½£ŗ
(1)ÄĘÓėĖ®·“Ó¦£ŗ_______________________________________________________________”£
(2)ĀČĘųĶØČėĖ®ÖŠ£ŗ_____________________________________________________________”£
(3)ĻņĒāŃõ»ÆÄĘČÜŅŗÖŠĶØČėÉŁĮæCO2£ŗ
________________________________________________________________________ӣ
(4)Ģ¼ĖįøĘÖŠµĪČė“×ĖįČÜŅŗ£ŗ
________________________________________________________________________ӣ
(5)ĀĮʬĶ¶ČėĒāŃõ»ÆÄĘČÜŅŗ£ŗ
________________________________________________________________________ӣ
(6)ĀČ»ÆĀĮČÜŅŗÖŠ¼Ó×ćĮæĢ¼ĖįĒāÄĘČÜŅŗ£ŗ
________________________________________________________________________ӣ
(7)FeCl3ČÜŅŗÓėCu·“Ó¦£ŗ
________________________________________________________________________ӣ
(8)ĖįŠŌĮņĖįŃĒĢśČÜŅŗÖŠ¼ÓČė¹żŃõ»ÆĒāČÜŅŗ£ŗ
________________________________________________________________________ӣ
(9)ŹµŃéŹŅÓĆMnO2ÓėÅØŃĪĖįÖĘČ”Cl2£ŗ
________________________________________________________________________ӣ
(10)NO2ČÜÓŚĖ®£ŗ_____________________________________________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
³£ĪĀŹ±£¬ĻĀĮŠø÷×éĄė×ÓÄÜ“óĮæ¹²“ęµÄŹĒ(””””)
A£®Pb2£«”¢K£«”¢NO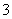 ”¢SO
ӢSO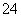
B£®Fe3£«”¢NH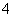 ”¢S2£”¢NO
”¢S2£”¢NO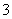
C£®Fe2£«”¢Na£«”¢Cl£”¢NO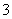
D£®H£«”¢Cl£”¢SO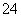 ”¢AlO
ӢAlO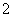
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
Īķö²ŃĻÖŲÓ°ĻģČĖĆĒµÄÉś»īÓė½”æµ”£Ä³µŲĒųµÄĪķö²ÖŠæÉÄÜŗ¬ÓŠČēĻĀæÉČÜŠŌĪŽ»śĄė×Ó£ŗNa£«”¢NH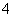 ”¢Mg2£«”¢Al3£«”¢SO
”¢Mg2£«”¢Al3£«”¢SO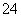 ”¢NO
”¢NO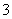 ”¢Cl£ ”£Ä³Ķ¬Ń§ŹÕ¼ÆĮĖøƵŲĒųµÄĪķö²£¬¾±ŲŅŖµÄŌ¤“¦ĄķŗóµĆŹŌŃłČÜŅŗ£¬Éč¼Ę²¢Ķź³ÉĮĖČēĻĀŹµŃé£ŗ
”¢Cl£ ”£Ä³Ķ¬Ń§ŹÕ¼ÆĮĖøƵŲĒųµÄĪķö²£¬¾±ŲŅŖµÄŌ¤“¦ĄķŗóµĆŹŌŃłČÜŅŗ£¬Éč¼Ę²¢Ķź³ÉĮĖČēĻĀŹµŃé£ŗ
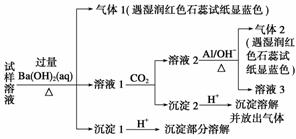
ŅŃÖŖ£ŗ3NO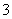 £« 8Al£«5OH££«2H2O
£« 8Al£«5OH££«2H2O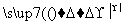 3NH3”ü£«8AlO
3NH3”ü£«8AlO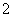
øł¾ŻŅŌÉĻµÄŹµŃé²Ł×÷ÓėĻÖĻó£¬øĆĶ¬Ń§µĆ³öµÄ½įĀŪ²»ÕżČ·µÄŹĒ(””””)
A£®ŹŌŃłÖŠæĻ¶Ø“ęŌŚNH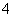 ”¢Mg2£«”¢SO
”¢Mg2£«”¢SO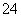 ŗĶNO
ŗĶNO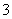
B£®ŹŌŃłÖŠŅ»¶Ø²»ŗ¬Al3£«
C£®ŹŌŃłÖŠæÉÄÜ“ęŌŚNa£«”¢Cl£
D£®øĆĪķö²ÖŠæÉÄÜ“ęŌŚNaNO3 ”¢NH4ClŗĶMgSO4
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŅŃÖŖŅŅĖį£ØHA£©µÄĖįŠŌ±Č¼×Ėį£ØHB£©Čõ,ŌŚĪļÖŹµÄĮæÅØ¶Č¾łĪŖ0.1 mol/LµÄNaAŗĶNaB»ģŗĻČÜŅŗÖŠ,ĻĀĮŠÅÅŠņÕżČ·µÄŹĒ£Ø £©
A.c(OH-)>c(HA)>c(HB)>c(H+) B.c(OH-)>c(A-)>c(B-)>c(H+)
C.c(OH-)>c(B-)>c(A-)>c(H+) D.c(OH-)>c(HB)>c(HA)>c(H+)
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com