
ijŹµŃ銔×éÓĆ¹¤Ņµ·ĻĘś¹ĢĢå£ØÖ÷ŅŖ³É·ÖĪŖCu2SŗĶFe2O3£©ÖʱøÓŠ¹ŲĪļÖŹ£¬ÕūøöĮ÷³ĢČēĻĀĶ¼ĖłŹ¾”£Ēė»Ų“š£ŗ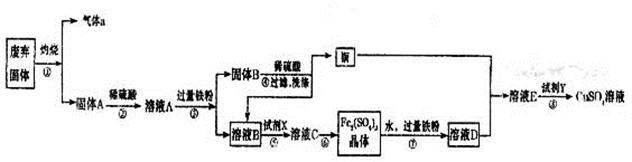
£Ø1£©ĘųĢåaµÄ»ÆѧŹ½ĪŖ ”£
£Ø2£©ČÜŅŗB¼ÓČėĮņĖįĖį»ÆŗóŌŁ¼ÓČėŹŹŅĖŃõ»Æ¼ĮXµĆµ½ČÜŅŗC£¬Š“³öøĆ·“Ó¦µÄĄė×Ó·½³ĢŹ½ ”£
£Ø3£©ÖʱøĮņĖįĶČÜŅŗ”£³£ĪĀĻĀ£¬O2”¢Ķ·ŪŗĶĻ”ĮņĖįČżÕßŌŚŅ»Ęš£¬¼øŗõ²»·“Ó¦£¬µ±¼ÓČėČÜŅŗDŗó£¬Ėę¼“Éś³ÉĮņĖįĶ”£¾ĄķŌÄ׏ĮĻ·¢ĻÖFeSO4¶ŌĶµÄŃõ»ÆĘš“ß»Æ×÷ÓĆ”£
A.µŚŅ»²½·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ£ŗ4Fe2£«£«O2£«4H£«=4Fe3£«£«2H2O£¬ŌņµŚ¶ž²½·“Ó¦µÄ¹ł×Ó·½³ĢŹ½ĪŖ ”£
B.¢ß²Ł×÷ÖŠ£¬ÅäÖĘFe2(SO4)3ČÜŅŗŹ±Ó¦×¢Ņā ”£
£Ø4£©²Ł×÷¢ąµÄÄæµÄŹĒµĆµ½½Ļ“æµÄĮņĖįĶČÜŅŗ”£¼ÓČėŹŹŅĖŹŌ¼ĮYµ÷½ŚpHÖĮĢśŌŖĖŲČ«²æ³Įµķ£ØĄė×ÓÅØ¶ČŠ”ÓŚ10£5mol/L£©£¬Č»ŗóŌŁ¹żĀĖ£¬ÅØĖõ”¢½į¾§µČ£¬ŌņpHÖĮÉŁµ÷½ŚĪŖ_____”£
ŅŃÖŖ£ŗKsp[Cu(OH)2]”Ö1”Į10£22£¬Ksp[Fe(OH)2] ”Ö1”Į10£16£¬Ksp[Fe(OH)3] ”Ö1”Į10£38
£Ø5£©æĘѧ¼Ņ·¢ĻÖÄÉĆ×¼¶µÄCu2OŌŚĢ«Ńō¹āÕÕÉäĻĀæÉŅŌ“߻ƷֽāĖ®”£
A.Ņ»¶ØĪĀ¶ČĻĀ£¬ŌŚ2LĆܱÕČŻĘ÷ÖŠ¼ÓČėÄÉĆ×¼¶Cu2O£¬ĶØČė2molĖ®ÕōĘų£¬·¢ÉśČēĻĀ·“Ó¦£ŗ
2H2O(g)£½2H2(g)£«O2(g) ”÷H£½£«484kJ/mol
20minÄ©²āµĆn(O2)£½0.16mol£¬ŌņÕā¶ĪŹ±¼äµÄ·“Ó¦ĖŁĀŹ¦Ō(H2)£½_________£»øĆĪĀ¶ČĻĀ£¬“Ė·“Ó¦µÄĘ½ŗā³£Źż±ķ“ļŹ½K£½___________________”£
B.ŅŃÖŖ£ŗ2Cu2O(s)£«O2(g)£½4CuO(s) ”÷H£½£292kJ/mol
2C(s)£«O2(g)£½2CO(g) ”÷H£½£221kJ/mol
ĒėŠ“³öĢæ·Ū»¹ŌCuO(s)ÖʱøCu2O(s)µÄČČ»Æѧ·½³ĢŹ½_________________”£
£Ø³żČ„×¢Ć÷Ķā£¬ĆææÕ2·Ö£¬¹²14·Ö£©£Ø1£©SO2(1·Ö)
£Ø2£©2Fe2£«£«H2O2£«2H£«£½2Fe3£«£«2H2O»ņ4Fe2£«£«O2£«4H£«£½4Fe3£«£«2H2O
£Ø3£©2Fe3£«£«Cu£½2Fe2£«£«Cu2£«£»ĻņÅäÖĘČÜŅŗÖŠ¼ÓČėÉŁĮæµÄĮņĖį·ĄÖ¹Ė®½ā»ņ½«Fe2(SO4)3ČܽāŌŚĻ”ĮņĖįÖŠ£¬ŌŁ¼ÓĖ®Ļ”ŹĶ £Ø4£©3 £Ø5£©0.008mol/(L”¤min)£Øµ„Ī»Õ¼1·Ö£©£»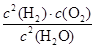 £Ø1·Ö£©£»
£Ø1·Ö£©£»
2CuO(s)£«C(s)£½CO(g)£«Cu2O(s) ”÷H£½£«35.5 kJ/mol
½āĪöŹŌĢā·ÖĪö£ŗ£Ø1£©Cu2S×ĘÉÕÉś³ÉSO2ŗĶĶ£¬·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒCu2S£«O2 2Cu£«SO2”ü£¬Ņņ“ĖĘųĢåaŹĒSO2”£
2Cu£«SO2”ü£¬Ņņ“ĖĘųĢåaŹĒSO2”£
£Ø2£©¹ĢĢåAŹĒŃõ»ÆĢśŗĶĶµÄ»ģŗĻĪļ£¬¼ÓČėĻ”ĮņĖį¹ĢĢåČܽāÉś³ÉĮņĖįĢś£¬½ų¶ųĮņĖįĢśÓÖČܽāĶ£¬ĖłŅŌČÜŅŗAŹĒĮņĖįĢś”¢ĮņĖįŃĒĢś”¢ĮņĖįĶŅŌ¼°ĮņĖįµÄ»ģŗĻŅŗ”£¼ÓČė¹żĮæµÄĢś·ŪŗóÉś³ÉĮņĖįŃĒĢśŗĶĶ£¬ĖłŅŌ¹ĢĢåBŹĒĶŗĶĢśµÄ»ģŗĻĪļ£¬ČÜŅŗBŹĒĮņĖįŃĒĢś”£ŅŖµĆµ½ĮņĖįĢś¾§Ģ壬ŌņŠčŅŖ½«ĮņĖįŃĒĢśŃõ»ÆÉś³ÉĮņĖįĢś£¬Ņņ“ĖŃõ»Æ¼ĮXæÉŅŌŹĒĖ«ŃõĖ®»ņŃõĘų£¬ÓŠ¹ŲµÄĄė×Ó·½³ĢŹ½ŹĒ2Fe2£«£«H2O2£«2H£«£½2Fe3£«£«2H2O»ņ4Fe2£«£«O2£«4H£«£½4Fe3£«£«2H2O”£
£Ø3£©ÓÉÓŚĢśĄė×Ó¾ßÓŠŃõ»ÆŠŌ£¬ÄÜ°ŃĶŃõ»ÆÉś³ÉĶĄė×Ó£¬¶ųĢśĄė×ÓÓÖ±»»¹ŌÉś³ÉŃĒĢśĄė×Ó£¬Ņņ“ĖµŚ¶ž²½·“Ó¦µÄĄė×Ó·½³ĢŹ½ŹĒ2Fe3£«£«Cu£½2Fe2£«£«Cu2£«£»ÓÉÓŚĢśĄė×ÓŅ×Ė®½āÉś³ÉĒāŃõ»ÆĢś£¬ĖłŅŌÅäÖĘĮņĖįĢśČÜŅŗŹ±£¬ŅŖ·ĄÖ¹ĢśĄė×ÓĖ®½ā£¬Ņņ“ĖÕżČ·µÄ²Ł×÷Ó¦øĆŹĒĻņÅäÖĘČÜŅŗÖŠ¼ÓČėÉŁĮæµÄĮņĖį·ĄÖ¹Ė®½ā»ņ½«Fe2(SO4)3ČܽāŌŚĻ”ĮņĖįÖŠ£¬ŌŁ¼ÓĖ®Ļ”ŹĶ”£
£Ø4£©ČÜŅŗEŹĒĮņĖįĶŗĶĮņĖįŃĒĢśµÄ»ģŗĻŅŗ£¬øł¾ŻČܶȻż³£ŹżæÉÖŖ£¬ŅŖµĆµ½ĮņĖįĶČÜŅŗ£¬ŠčŅŖ³żČ„ŃĒĢśĄė×Ó”£ÓÉÓŚĒāŃõ»ÆŃĒĢśµÄČܶȻż³£Źż“óÓŚĒāŃõ»ÆĶµÄ£¬µ«ĒāŃõ»ÆĢśµÄČܶȻż³£ŹżŠ”ÓŚĒāŃõ»ÆĶµÄ£¬ĖłŅŌÓ¦øĆ¼ÓČėŃõ»Æ¼Į½«ŃĒĢśĄė×ÓŃõ»Æ³ÉĢśĄė×Ó”£øł¾ŻĒāŃõ»ÆĢśµÄČܶȻż³£ŹżæÉÖŖ£¬ŅŖµ÷½ŚpHÖĮĢśŌŖĖŲČ«²æ³Įµķ£ØĄė×ÓÅØ¶ČŠ”ÓŚ10£5mol/L£©£¬ČÜŅŗÖŠµÄOH£Ó¦øĆĪŖ £½10£11mol/L£¬ĖłŅŌpHÖĮÉŁµ÷½ŚĪŖ3”£
£½10£11mol/L£¬ĖłŅŌpHÖĮÉŁµ÷½ŚĪŖ3”£
£Ø5£©20minÄ©²āµĆn(O2)£½0.16mol£¬Ōņøł¾Ż·½³ĢŹ½æÉÖŖ£¬Éś³ÉĒāĘųŹĒ0.16mol”Į2£½0.32mol£¬ĘäÅØ¶ČŹĒ0.32mol”Ā2L£½0.16mol/L£¬Ņņ“ĖĒāĘųµÄ·“Ó¦ĖŁĀŹ¦Ō(H2)£½0.16mol/L”Ā20min£½0.008mol/(L”¤min)”£»ÆŃ§Ę½ŗā³£ŹżŹĒŌŚŅ»¶ØĢõ¼žĻĀ£¬µ±æÉÄę·“Ó¦“ļµ½Ę½ŗāדĢ¬Ź±£¬Éś³ÉĪļÅØ¶ČµÄĆŻÖ®»żŗĶ·“Ó¦ĪļÅØ¶ČµÄĆŻÖ®»żµÄ±ČÖµ£¬Ņņ“Ėøł¾Ż·½³ĢŹ½æÉÖŖøĆĪĀ¶ČĻĀ£¬“Ė·“Ó¦µÄĘ½ŗā³£Źż±ķ“ļŹ½K£½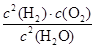 £»øł¾Ż·“Ó¦¢Ł£ŗ2Cu2O(s)£«O2(g)£½4CuO(s) ”÷H£½£292kJ/molŗĶ·“Ó¦¢Ś£ŗ2C(s)£«O2(g)£½2CO(g) ”÷H£½£221kJ/mol²¢ŅĄ¾ŻøĒĖ¹¶ØĀÉæÉÖŖ£¬£Ø¢Ś£¢Ł£©”Ā2¼“µĆµ½·“Ó¦2CuO(s)£«C(s)£½CO(g)£«Cu2O(s)£¬ĖłŅŌøĆ·“Ó¦µÄ·“Ó¦ČČ”÷H£½£Ø£221kJ/mol£«292kJ/mol£©”Ā2£½£«35.5 kJ/mol”£
£»øł¾Ż·“Ó¦¢Ł£ŗ2Cu2O(s)£«O2(g)£½4CuO(s) ”÷H£½£292kJ/molŗĶ·“Ó¦¢Ś£ŗ2C(s)£«O2(g)£½2CO(g) ”÷H£½£221kJ/mol²¢ŅĄ¾ŻøĒĖ¹¶ØĀÉæÉÖŖ£¬£Ø¢Ś£¢Ł£©”Ā2¼“µĆµ½·“Ó¦2CuO(s)£«C(s)£½CO(g)£«Cu2O(s)£¬ĖłŅŌøĆ·“Ó¦µÄ·“Ó¦ČČ”÷H£½£Ø£221kJ/mol£«292kJ/mol£©”Ā2£½£«35.5 kJ/mol”£
æ¼µć£ŗæ¼²éŃõ»Æ»¹Ō·“Ó¦²śĪļÅŠ¶Ļ”¢·½³ĢŹ½ŹéŠ“”¢ĮņĖįĢśÅäÖĘ”¢ČܶȻż³£ŹżµÄÓŠ¹ŲÓ¦ÓĆ”¢·“Ó¦ĖŁĀŹµÄ¼ĘĖć”¢Ę½ŗā³£ŹżŅŌ¼°ČČ»Æѧ·½³ĢŹ½µÄŹéŠ“µČ


 ĆūŠ£æĪĢĆĻµĮŠ“š°ø
ĆūŠ£æĪĢĆĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ijæĪĶāŠ”×é¶ŌŅ»Š©½šŹōµ„ÖŹŗĶ»ÆŗĻĪļµÄŠŌÖŹ½ųŠŠŃŠ¾æ”£
£Ø1£©ĻĀ±ķĪŖ”°ĀĮÓėĀČ»ÆĶČÜŅŗ·“Ó¦”±ŹµŃé±ØøęµÄŅ»²æ·Ö£ŗ
| ŹµŃé²½Öč | ŹµŃéĻÖĻó |
| ¢Ł½«“ņÄ„¹żµÄĀĮʬ£Ø¹żĮ棩·ÅČėŅ»¶ØÅØ¶ČµÄCuCl2ČÜŅŗÖŠ”£ | ²śÉśĘųÅŻ£¬Īö³öŹčĖɵÄŗģÉ«¹ĢĢ壬ČÜŅŗÖš½„±äĪŖĪŽÉ«”£ |
| ¢Ś·“Ó¦½įŹųŗó·ÖĄė³öČÜŅŗ±øÓĆ”£ | |
| ¢ŪŗģÉ«¹ĢĢåÓĆÕōĮóĖ®Ļ“µÓŗó£¬ÖĆÓŚ³±ŹŖæÕĘųÖŠ”£ | Ņ»¶ĪŹ±¼äŗó¹ĢĢåÓÉŗģÉ«±äĪŖĀĢÉ«[ŹÓĘäÖ÷ŅŖ³É·ÖĪŖCu2(OH)2CO3]”£ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ĀĮĶĮæó(Ö÷ŅŖ³É·ÖĪŖAl2O3£¬»¹ÓŠÉŁĮæŌÓÖŹ)ŹĒĢįČ”Ńõ»ÆĀĮµÄŌĮĻ”£ĢįČ”Ńõ»ÆĀĮµÄ¹¤ŅÕĮ÷³ĢČēĻĀ£ŗ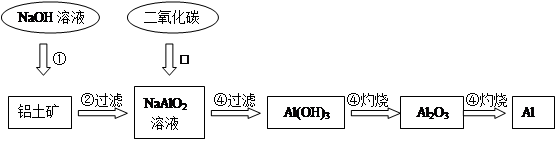
£Ø1£©ĒėÓĆĄė×Ó·½³ĢŹ½±ķŹ¾ŅŌÉĻ¹¤ŅÕĮ÷³ĢÖŠµŚ¢Ł²½·“Ó¦£ŗ_______ _______”£
£Ø2£©Š“³öŅŌÉĻ¹¤ŅÕĮ÷³ĢÖŠµŚ¢Ū²½·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ______ ___________”£
£Ø3£©½šŹōĀĮÓėŃõ»ÆĢś»ģŗĻŌŚøßĪĀĻĀ£¬»į·¢Éś¾ēĮŅµÄ·“Ó¦”£øĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½_____________”£Ēė¾ŁŅ»ĄżøĆ·“Ó¦µÄÓĆĶ¾________________”£
£Ø4£©µē½āČŪČŚŃõ»ÆĀĮÖĘČ”½šŹōĀĮ£¬ČōÓŠ0.9molµē×Ó·¢Éś×ŖŅĘ£®ĄķĀŪÉĻÄܵƵ½½šŹōĀĮµÄÖŹĮæŹĒ________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ĒāŃõ»ÆÄĘČÜŅŗ“¦ĄķĀĮĶĮæó²¢¹żĀĖ£¬µĆµ½ŗ¬ĀĮĖįÄʵÄČÜŅŗ”£ĻņøĆČÜŅŗÖŠĶØČė¶žŃõ»ÆĢ¼£¬ÓŠĻĀĮŠ·“Ó¦£ŗ 2NaAl(OH)4+CO2”ś2Al(OH)3”ż +Na2CO3+H2O
£Ø1£©ÉĻŹöĪåÖŠĪļÖŹÖŠ·Šµć×īµĶĪļÖŹµÄ½į¹¹Ź½ĪŖ______________£¬ÓÉÉĻŹöĪļÖŹÖŠµÄĮ½ÖÖŌŖĖŲ°“Ō×ÓøöŹż±Č1£ŗ1ŠĪ³ÉµÄĄė×Ó»ÆŗĻĪļµÄµē×ÓŹ½ĪŖ__________________£ØŠ“Ņ»Ąż£©
£Ø2£©AlŌŖĖŲµÄµ„ÖŹÓŠŠķ¶ą²»Ķ¬ÓŚĘäĖū½šŹōµÄĢŲŠŌ£¬ĒėĮŠ¾Ł2Ąż£ØŅ²æÉŅŌÓĆ»Æѧ·½³ĢŹ½±ķŹ¾£©
______________________”¢__________________________________£®
£Ø3£©ĒāÓŠ3ÖÖĪȶØĶ¬Ī»ĖŲ£¬Hė”¢ Dė®”¢ Tė°£¬·Ö±šĪŖ·į¶Ča”¢b”¢c£¬Ōņ¼ĘĖćĒāŌŖĖŲµÄ½üĖĘĻą¶ŌŌ×ÓÖŹĮæµÄ±ķ“ļŹ½ĪŖ______________________________________________£®
¼×ČĻĪŖHæÉŅŌÅÅŌŚÖÜĘŚ±ķ¢ńA×壬Ņ²æÉŅŌÅÅŌŚ¢÷A×壻¶ųŅŅĶ¬Ń§ČĻĪŖHŅ²æÉŅŌÓėĢ¼Ņ»Ńł£¬ÅÅŌŚ¢ōA×壬ŅŅĶ¬Ń§µÄĄķÓÉŹĒ__________________________________________________”£
£Ø4£©¼ŗÖŖĶØČė¶žŃõ»ÆĢ¼336 L(±ź×¼×“æöĻĀ)£¬ĄķĀŪÉĻÉś³ÉAl(OH)3 ________________mol,
Źµ¼ŹÉĻÉś³É24 mol Al(OH)3ŗĶ15 mol Na2CO3£¬Al(OH)3±ČĄķĀŪÉĻŅŖÉŁµÄŌŅņŹĒ£ŗ________________________________________________________£®
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
Ģś¼°Ęä»ÆŗĻĪļÓŠÖŲŅŖÓĆĶ¾£¬Čē¾ŪŗĻĮņĖįĢś[Fe2(OH)n£ØSO4)3-n/2]mŹĒŅ»ÖÖŠĀŠĶøߊ§µÄĖ®“¦Ąķ»ģÄż¼Į£¬¶ųøßĢśĖį¼Ų£ØĘäÖŠĢśµÄ»ÆŗĻ¼ŪĪŖ+6£©ŹĒŅ»ÖÖÖŲŅŖµÄɱ¾śĻū¶¾¼Į£¬Ä³»ÆѧĢ½¾æŠ”×éÉč¼ĘČēĻĀ·½°øÖʱøÉĻŹöĮ½ÖÖ²śĘ·£ŗ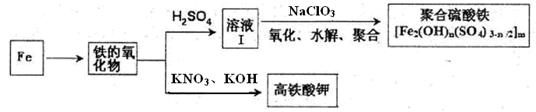
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©¼ģŃé¹ĢĢåĢśµÄŃõ»ÆĪļÖŠĢśµÄ»ÆŗĻ¼Ū£¬Ó¦Ź¹ÓƵďŌ¼ĮŹĒ £ØĢī±źŗÅ£©
| A£®Ļ”ĮņĖį | B£®Ļ”ĻõĖį | C£®KSCNČÜŅŗ | D£®ĖįŠŌøßĆĢĖį¼ŲČÜŅŗ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ĮŖ¼ī·Ø£ØŗņŹĻÖĘ¼ī·Ø£©ŗĶ°±¼ī·ØµÄÉś²śĮ÷³Ģ¼ņŅŖ±ķŹ¾ČēĻĀĶ¼£ŗ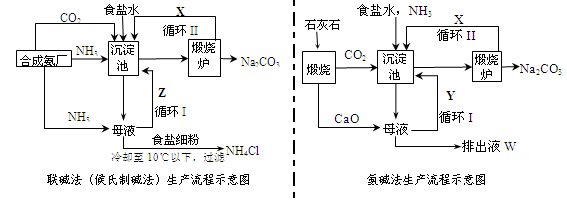
£Ø1£©Į½ÖÖ·½·ØµÄ³Įµķ³ŲÖŠ¾ł·¢ÉśµÄ·“Ó¦»Æѧ·½³ĢŹ½ĪŖ_____________________________”£
£Ø2£©Čō³Įµķ³Ųŗ¬800.00 mol NH3µÄĖ®ČÜŅŗÖŹĮæĪŖ54.00 kg£¬ĻņøĆČÜŅŗĶØČė¶žŃõ»ÆĢ¼ÖĮ·“Ó¦ĶźČ«£¬¹żĀĖ£¬µĆµ½ĀĖŅŗ31.20kg£¬ŌņNH4HCO3µÄ²śĀŹĪŖ______________£„”£
£Ø3£©ŌŚ°±¼ī·ØÉś²ś¹ż³ĢÖŠ°±ŅŖŃ»·Ź¹ÓĆ£¬µ«²»ŠčŅŖ²¹³ä£¬ŌŚÄøŅŗÖŠ¼ÓÉśŹÆ»ŅĒ°ĻČŅŖ¼ÓČȵÄŌŅņŹĒ ___ ”£
£Ø4£©øł¾ŻĮŖ¼ī·ØÖŠ“ÓĀĖŅŗÖŠĢįČ”ĀČ»Æļ§¾§ĢåµÄ¹ż³ĢĶĘ²ā£¬ĖłµĆ½įĀŪÕżČ·ŹĒ_______£ØŃ”Ģī±ąŗÅ£©”£
a£®³£ĪĀŹ±ĀČ»Æļ§µÄČܽā¶Č±ČĀČ»ÆÄĘŠ”
b£®ĶØČė°±ĘųÄÜŌö“óNH4+µÄÅØ¶Č£¬Ź¹ĀČ»Æļ§øü¶ąĪö³ö
c£®¼ÓČėŹ³ŃĪĻø·ŪÄÜĢįøßNa+µÄÅØ¶Č£¬ Ź¹NaHCO3½į¾§Īö³ö
d£®ĶØČė°±ĘųÄÜŹ¹NaHCO3×Ŗ»ÆĪŖNa2CO3£¬ĢįøßĪö³öµÄNH4Cl“æ¶Č
£Ø5£©ĮŖ¼ī·ØĻą±ČÓŚ°±¼ī·Ø£¬ĀČ»ÆÄĘĄūÓĆĀŹ“Ó70%Ģįøßµ½90%ŅŌÉĻ£¬Ö÷ŅŖŹĒÉč¼ĘĮĖŃ»·¢ń£¬ĮŖ¼ī·ØµÄĮķŅ»ĻīÓŵćŹĒ__________________________________________________”£
£Ø6£©“Ó³Įµķ³ŲĪö³öµÄ¾§Ģåŗ¬ÓŠNaClŌÓÖŹ£¬Ä³Ķ¬Ń§ŌŚ²ā¶ØĘäNaHCO3µÄŗ¬ĮæŹ±£¬³ĘČ”5.000gŹŌŃł£¬ÅäÖĘ³É100mLČÜŅŗ£¬ÓƱź×¼ŃĪĖįČÜŅŗµĪ¶Ø£ØÓĆ¼×»ł³Č×öÖøŹ¾¼Į£©£¬²ā¶ØŹż¾Ż¼ĒĀ¼ČēĻĀ£ŗ
| µĪ¶Ø“ĪŹż | “ż²āŅŗ£ØmL£© | 0.6000mol/LŃĪĖįČÜŅŗµÄĢå»ż£ØmL£© | |
| ³õ¶ĮŹż | ÖÕ¶ĮŹż | ||
| µŚŅ»“Ī | 20.00 | 1.00 | 21.00 |
| µŚ¶ž“Ī | 20.00 | ČēÓŅĶ¼¢ń | ČēÓŅĶ¼¢ņ |
 ĻŌŹ¾ĻūŗĵÄŃĪĖįČÜŅŗĢå»żĪŖ ”£
ĻŌŹ¾ĻūŗĵÄŃĪĖįČÜŅŗĢå»żĪŖ ”£²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ijŃõ»ÆĢśŃłĘ·ÖŠŗ¬ÓŠÉŁĮæµÄFeSO4ŌÓÖŹ”£Ä³Ķ¬Ń§ŅŖ²ā¶ØĘäÖŠĢśŌŖĖŲµÄÖŹĮæ·ÖŹż£¬ĖūÉč¼ĘĮĖČēĻĀ·½°ø½ųŠŠ²ā¶Ø£¬²Ł×÷Į÷³ĢĪŖ£ŗ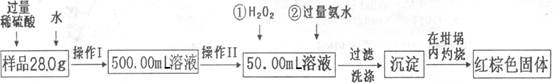
Ēėøł¾ŻĮ÷³Ģ»Ų“š£ŗ
£Ø1£©²Ł×÷IÖŠÅäÖĘČÜŅŗŹ±£¬ĖłÓƵ½µÄ²£Į§ŅĒĘ÷³żÉÕ±”¢ĮæĶ²”¢²£Į§°ō”¢½ŗĶ·µĪ¹ÜŅŌĶā£¬»¹±ŲŠėÓŠ (ĢīŅĒĘ÷Ćū³Ę£©”£
£Ø2£©²Ł×÷IIÖŠ±ŲŠėÓƵ½µÄŅĒĘ÷ŹĒ ”£
| A£®50mLĮæĶ² | B£®100mLĮæĶ² |
| C£®50mLĖįŹ½µĪ¶Ø¹Ü | D£®50mL¼īŹ½µĪ¶Ø¹Ü |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĪŹ“šĢā
£Ø14·Ö£©Ģś”¢ĀĮ”¢Ķ”¢¹č¼°ĘäŗĻ½š²ÄĮĻŌŚÉś²śÉś»ī֊ӊ׏ć·ŗµÄÓ¦ÓĆ”£Ēė»Ų“šĻĀĮŠÓŠ¹ŲĪŹĢā”£
£Ø1£©ÄæĒ°ŅŃŅ±Į¶³ö“æ¶Č“ļ99£®9999£„µÄĢś”£ĻĀĮŠ¹ŲÓŚ“æĢśµÄŠšŹö“ķĪóµÄŹĒ
£ØĢī×ÖÄø£©”£
| A£®Ó²¶Č±ČøÖŠ”£¬ČŪµć±ČøÖøß | B£®²»ÄÜÓėŃĪĖį·“Ó¦ |
| C£®Óė²»ŠāøֳɷÖĻąĶ¬ | D£®ŌŚĄäµÄÅØĮņĖįÖŠ¶Ū»Æ |
 6Cu+SO2”ü£¬øĆ·“Ó¦µÄŃõ»Æ¼ĮŹĒ””””””””””
6Cu+SO2”ü£¬øĆ·“Ó¦µÄŃõ»Æ¼ĮŹĒ”””””””””” 
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
ijŠ”×éĶعżŹµŃéŃŠ¾æNa2O2ÓėĖ®µÄ·“Ó¦?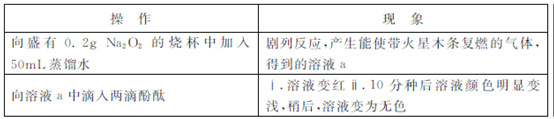
£Ø1£©Na2O2ÓėĖ®·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ ?
£Ø2£©¢¢ÖŠČÜŅŗĶŹÉ«æÉÄÜŹĒČÜŅŗaÖŠ“ęŌŚ½Ļ¶ąµÄH2O2,H2O2Óė·ÓĢŖ·¢ÉśĮĖ·“Ó¦?
¢ń£®¼×Ķ¬Ń§ĶعżŹµŃéÖ¤ŹµĮĖH2O2µÄ“ęŌŚ:ȔɣĮæČÜŅŗa,¼ÓČėŹŌ¼Į (Ģī»ÆѧŹ½)£¬ÓŠĘųĢå²śÉś?
¢ņ£®ŅŅĶ¬Ń§²éŌÄ׏ĮĻ»ńĻ¤:ÓĆKMnO4(±»»¹ŌĪŖ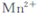 )æÉŅŌ²ā¶ØH2O2µÄŗ¬Įæ?
)æÉŅŌ²ā¶ØH2O2µÄŗ¬Įæ?
Č”3mLČÜŅŗaĻ”ŹĶÖĮ15mL,ÓĆĻ”H2SO4Ėį»Æ,ŌŁÖšµĪ¼ÓČė0£®0045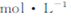 KMnO4ČÜŅŗ,²śÉśĘųĢå,ČÜŅŗĶŹÉ«ĖŁĀŹæŖŹ¼½ĻĀżŗó±äæģ,ÖĮÖÕµćŹ±¹²ĻūŗÄ10mL KMnO4ČÜŅŗ?
KMnO4ČÜŅŗ,²śÉśĘųĢå,ČÜŅŗĶŹÉ«ĖŁĀŹæŖŹ¼½ĻĀżŗó±äæģ,ÖĮÖÕµćŹ±¹²ĻūŗÄ10mL KMnO4ČÜŅŗ?
¢ŁKMnO4ÓėH2O2·“Ó¦µÄĄė×Ó·½³ĢŹ½ŹĒ ?
¢ŚČÜŅŗaÖŠ ?
?
¢ŪČÜŅŗĶŹÉ«ĖŁĀŹæŖŹ¼½ĻĀżŗó±äæģµÄŌŅņæÉÄÜŹĒ ?
£Ø3£©ĪŖĢ½¾æĻÖĻó¢¢²śÉśµÄŌŅņ,Ķ¬Ń§ĆĒ¼ĢŠų½ųŠŠĮĖČēĻĀŹµŃé:
¢ń£®ĻņH2O2ČÜŅŗÖŠµĪČėĮ½µĪ·ÓĢŖ,Õńµ“,¼ÓČė5µĪ0£®1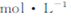 NaOHČÜŅŗ,ČÜŅŗ±äŗģÓÖŃøĖŁ±äĪŽÉ«ĒŅ²śÉśĘųĢå,10·ÖÖÓŗóČÜŅŗ±äĪŽÉ«,øĆ¹ż³ĢĪŽĆ÷ĻŌČČŠ§Ó¦?
NaOHČÜŅŗ,ČÜŅŗ±äŗģÓÖŃøĖŁ±äĪŽÉ«ĒŅ²śÉśĘųĢå,10·ÖÖÓŗóČÜŅŗ±äĪŽÉ«,øĆ¹ż³ĢĪŽĆ÷ĻŌČČŠ§Ó¦?
¢ņ£®Ļņ0£®1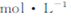 NaOHČÜŅŗÖŠµĪČėĮ½µĪ·ÓĢŖµÄ,Õńµ“,ČÜŅŗ±äŗģ,10·ÖÖÓŗóČÜŅŗŃÕÉ«ĪŽĆ÷ĻŌ±ä»Æ;ĻņøĆČÜŅŗÖŠĶØČėŃõĘų,ČÜŅŗŃÕÉ«ĪŽĆ÷ĻŌ±ä»Æ?“ÓŹµŃé¢ńŗĶ¢ņÖŠ£¬æÉµĆ³öµÄ½įĀŪŹĒ ?
NaOHČÜŅŗÖŠµĪČėĮ½µĪ·ÓĢŖµÄ,Õńµ“,ČÜŅŗ±äŗģ,10·ÖÖÓŗóČÜŅŗŃÕÉ«ĪŽĆ÷ĻŌ±ä»Æ;ĻņøĆČÜŅŗÖŠĶØČėŃõĘų,ČÜŅŗŃÕÉ«ĪŽĆ÷ĻŌ±ä»Æ?“ÓŹµŃé¢ńŗĶ¢ņÖŠ£¬æÉµĆ³öµÄ½įĀŪŹĒ ?
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com